12V batteries are classified by material and can be divided into: 12V lithium battery, 12V lead-acid battery, 12V nickel-metal hydride nickel-cadmium battery, 12V alkaline battery.
12V lithium battery
What is a 12V lithium battery
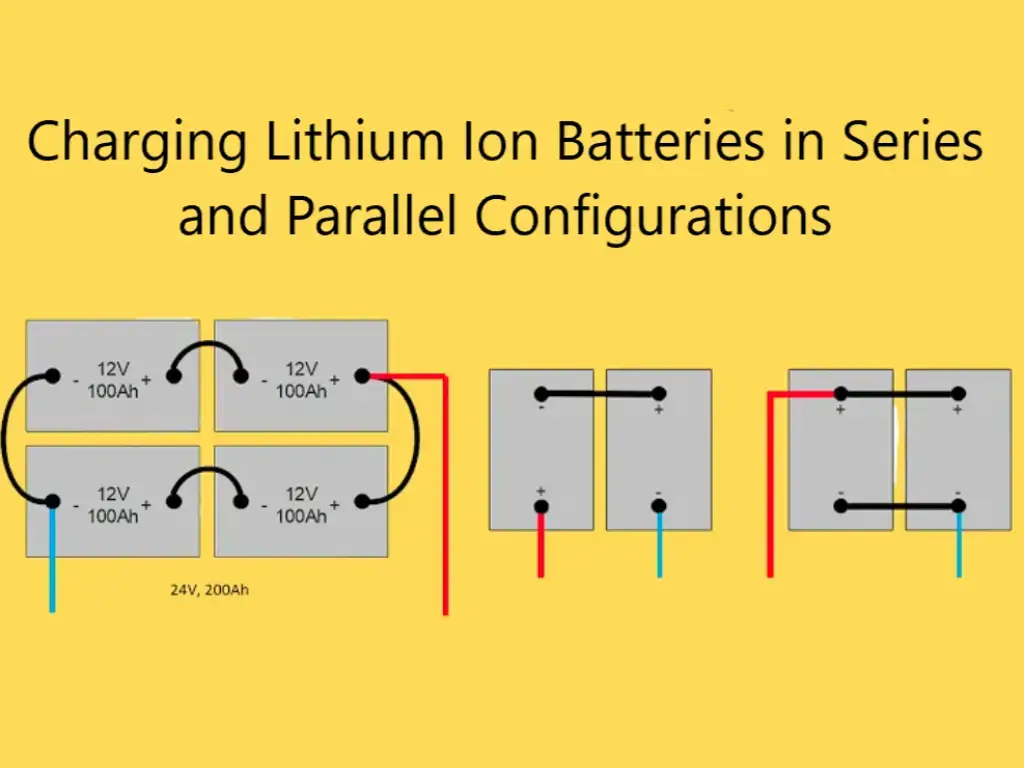
12v lithium batteries are connected in series with 3 or 4 lithium batteries. The combined battery pack, the capacity of the battery is determined according to the capacity of a single cell, or the capacity of the batteries connected in parallel, it is a new type of safe and environmentally friendly battery, the voltage of a single lithium battery after being fully charged is 4.2V, under normal circumstances Also called 4V, the voltages of the batteries in series add up, 4V/battery * 3 batteries = 12V, which is what we call a 12V lithium battery.
12V lithium battery parameters
- Combination: ICR18500-3S2P
- Nominal voltage: 11.1V
- Discharge voltage: 11.1V-12.6V
- Conventional discharge current is: 0-2A, large capacity is 3-4A
- Nominal capacity: in Mah A, available in various sizes
- Standard continuous discharge current: 0.2C
- Maximum continuous discharge current: 1C
- Working temperature: charging: 0~45℃
- Discharge: -20~60℃
- Product size: MAX 3956.699mm
- Internal resistance of finished product: ≤280mΩ
- Standard weight:
- Protection board: IC-S8254AAJ+MOS-AO4409
- Lead model: JST-VHR-2P positive plug UL1007/24# wire, wire length 100mm
- Protection parameters:
- Overcharge protection voltage/4.35±0.025V per string
- Over-discharge protection voltage 2.4±0.08V
- Overcurrent value: 10~25A
What are the types of 12v lithium-ion batteries?
12V lithium-ion batteries mainly have three types of outer packaging types: soft pack, steel shell, and square aluminum shell, while lithium battery cell voltages mainly include 3.2V and 3.7V.
The 12V lithium-ion battery composed of 3.2V cells belongs to the lithium iron phosphate battery pack, which is realized by using 4 cells in series;
3.7V cells are generally composed of polymer or ternary lithium-ion batteries to form 12V lithium-ion batteries, which are realized by using 4 cells in series and then adding a battery protection board to reduce the voltage.
Click to Buy 3.2V batteries
12V lithium-ion batteries are also divided into high-rate types and conventional types. Conventional types of batteries are mostly used in electronic equipment that does not require high-current discharge to provide stable low-current discharge. Usually, the battery capacity and service life are relatively high. High-rate batteries are mostly used in some high-current discharge equipment, and the discharge size varies according to the actual application. Such batteries have relatively high performance requirements and are correspondingly more expensive in price.
12V lithium-ion batteries are also divided into low temperature batteries, high temperature batteries and normal temperature batteries. Most of the batteries on the market are low temperature and can only be used in low temperature range, high temperature can only be used in high temperature range, and the span of use temperature range is not very large. , such as low-temperature batteries, if the specified operating temperature is generally -40 ℃ to 50 ℃, too high and too low will cause damage to the performance of the battery.
12V lithium battery is mainly used in which fields
As a common type of lithium battery, lithium battery has a wide range of applications. Many electronic devices choose 12V lithium battery as the power source. Many friends are particularly concerned about the specific application of 12V lithium battery. The preliminary statistics are as follows:
- Solar lighting industry, such as solar street lights, solar insecticidal lights, solar garden lights, solar energy storage power, etc.;
- In the electric toy industry, such as electric remote control cars, electric robots, etc., many electric toys also choose 12V lithium batteries;
- Mobile lighting industry, such as xenon lamps, high-power LED flashlights, diving lights, searchlights, etc.;
- The field of electric tools, such as electric screwdrivers, electric drills, electric scissors, etc.;
- The field of Bluetooth audio and loudspeaker;
- Handheld vacuum cleaner
- Electric sprayer
- Fishing machines, electric fish machines, etc.
- LED lamps and various electronic instruments and equipment, etc.
The application of 12V power supply is too extensive. The above are just a few examples. In fact, there are many other 12V lithium batteries that are also used.
Energy storage power lithium battery, 12V100Ah lithium iron phosphate battery, replace lead acid
100AH 12V Low Temperature Heated LiFePO4 Lithium Battery
Application field
Photovoltaic power generation, communication base station, island power generation, photovoltaic energy storage, solar energy storage system, wind-solar hybrid system, ships, electric boats, portable mobile power stations, electric vehicles, golf carts, forklifts, replacing 12V lead-acid batteries, etc.
How to charge 12V lithium battery?
The standard name of 12V lithium battery is 11.1V. It is named because the voltage is close to the lead-acid battery voltage of 12V. The 12V lithium battery consists of three groups or three batteries, and the maximum voltage is usually 12.6V; therefore, the charging voltage is 12.6V, normally within 10A The protection board equipped with the working current is the same port, charging and discharging the same IO interface (both charging line and discharge line)
Why don’t battery manufacturers leave the factory with 12v lithium battery cells?
13 years of lithium battery technology, AGV, RGV, medical, low temperature battery-based!
This is determined by the principle of lithium batteries. Lithium batteries are different from lead-acid batteries. The nominal voltage of lead-acid is 12V, the lithium iron lithium battery is 3.2V, and the ternary lithium battery is 3.7V.
12V lead-acid battery
- 12V lead-acid battery is a device that directly converts chemical energy into electrical energy. It is a rechargeable battery designed to be recharged through a reversible chemical reaction. It usually refers to a lead-acid battery, which is a kind of battery. It is a secondary battery.
12V lead-acid battery overview
The working principle of the battery: when charging, the internal active material is regenerated by using external electrical energy, and the electrical energy is stored as chemical energy.
It uses a lead substrate grid filled with spongy lead (also known as a lattice) as a negative electrode, a lead substrate grid filled with lead dioxide as a positive electrode, and dilute sulfuric acid with a density of 1.26–1.33g/mlg/ml is used as the electrolyte. When the battery is discharging, metal lead is the negative electrode, which undergoes an oxidation reaction to generate lead sulfate; lead dioxide is the positive electrode, which undergoes a reduction reaction to generate lead sulfate. When the battery is charged with direct current, elemental lead and lead dioxide are formed at the two poles, respectively. When the power source is removed, it returns to its pre-discharge state, forming a chemical battery. The lead-acid battery can be repeatedly charged and discharged. Its single-cell voltage is 2v. The battery is a battery pack composed of one or more cells, referred to as battery. The most common ones are 6v and 12v batteries. Others include 2v, 4v, 8v, 24v battery. For example, the battery used in the car (commonly known as the battery) is a battery pack of 6 lead-acid batteries connected in series to form a 12v battery.
For traditional dry-charged lead batteries (such as automobile dry-charged batteries, motorcycle dry-charged batteries, etc.), distilled water should be added after a period of use to keep the dilute sulfuric acid electrolyte at a density of about 1.28g/ml; it will be used until the end of its life. The addition of distilled water is no longer necessary.
The chemical reaction equation is as follows:
Overall reaction: pb(s) + pbo2(s) + 2 chi h2so4(aq) reversible symbol 2pbso4(s) + 2h2o(l)
When discharging: negative pb(s)-2e-+so42-(aq)=pbso4(s)
Positive pbo2(s)+2e-+so42-(aq)+4h+(aq)=pbso4(s)+2h2o(l)
Total pb(s)+pbo2(s)+2h2so4(aq)=2pbso4(s)+2h2o(l)
During charging Electrolytic cell
Cathode pbso4(s)+2e-=pb(s)+so42-(aq)
Anode pbso4(s)+2h2o(l)-2e-=pbo2(s)+so42-(aq)+4h+(a
Types of lead-acid batteries
In the early days, the battery case was open at the top, and the water could evaporate without hindrance. The acid mist also polluted the environment, and at the same time, it could not ensure that impurities did not enter the battery.
Batteries are now described as “flooded”.
In addition, there are “valve-regulated sealed” batteries, which are actually “maintenance-free”, so the problem of water replenishment is the focus. In the world they are called VRLA batteries.
The word “valve regulated seal” means something is absolute, however “flooded” means permission to open i.e. the battery cover is opened in some way, when the battery is closed by a plug of various designs, it is not guaranteed The outside and the inside of the battery are completely airtight.
Standard flooded batteries do of course require maintenance and therefore require pure water (such as distilled or deionized water) to be added from the top. Obviously the battery must be perforated in order to add water.
- As far as the valve regulated sealed battery is concerned, there is no need to add water holes, which will be discussed later.
- At present, the types of lead-acid batteries can be divided according to the type of positive plate and the form of the mechanism.
- The types of plates have been discussed in the article Plate Types for Lead-Acid Batteries.
- According to the form of structure, the single battery and the whole battery are distinguished:
- A single cell, judging by its name, represents a traditional classification form with a rated voltage of 2V.
- A monolithic battery (4V, 6V or even 12V for VRLA) means that there are several cells connected together in one battery case.
The following information can be obtained from the identification of the single battery and the whole battery:
— Is it a single battery or a whole battery
—Type (positive plate)
—Number of positive plates
—Nominal capacity (10 hour rate)
Low maintenance flooded batteries and maintenance-free VRLA batteries are distinguished according to whether they are used for maintenance or not:
- Low maintenance flooded batteries generally have the following forms according to the type of positive and negative plates:
- 1) The negative electrode is a paste-type electrode plate, and the positive electrode is a Plant-type electrode plate.
- This form has excellent high-current discharge performance, suitable for both seconds and hours of discharge, but is not suitable for cyclic use.
- 2) Positive and negative electrodes are paste-coated plates
- This form has excellent high-current discharge performance, and is mainly used for medium-to-high current discharge, which is more suitable for cyclic use than 1).
- 3) The negative electrode is a paste-type electrode plate, and the positive electrode is a tubular electrode plate.
- This form is suitable for long-term low to medium current discharge, suitable for cycling and has a long cycle life.
- Maintenance-free valve-regulated sealed battery
“Maintenance-free” means that no water needs to be added over the life of the battery, on the other hand means that the decomposition of water must be avoided or at least minimized.
However, water splitting cannot be completely avoided. The excess gas, usually just hydrogen, with very little oxygen under normal circumstances, can escape through the safety valve to balance the pressure inside and outside the battery. This valve (such as a rubber cap) keeps outside air from entering the interior of the battery. Therefore, having this device is one of the basic conditions for the battery to achieve maintenance-free.
- Internationally, the English letter “VRLA (Valve Regulated Lead Acid) battery” is used to represent the valve-regulated sealed lead-acid battery.
- How to design a “maintenance-free” battery, and what is the difference between it and the traditional low-maintenance battery?
- Let’s take a look at the diagram below, which gives the basic structure of VRLA:
The chemistry of a flooded battery during charge and discharge is the same, however it is also familiar that the electrolyte is immobilized in a colloid or absorbed in a fiberglass membrane to seal the battery.
In this way, there are gel batteries or adsorption batteries. Therefore, fixed electrolyte is one of the two basic conditions for VRLA batteries to be maintenance-free.
During overcharge, the current is used almost exclusively to generate oxygen at the positive electrode and water at the negative electrode, a process that is exothermic (produces heat!) and almost completely avoids the formation of hydrogen at the negative electrode. Hydrogen generation at the negative electrode can be suppressed only if the oxygen gas can easily reach the negative electrode from the positive electrode (the oxygen diffuses through the colloidal or glass fiber membrane and the internal space of the cell after leaving the electrolyte).
- For liquid electrolytes, this process is more difficult. The oxygen circulation is stopped while hydrogen is formed. In order to suppress the formation of hydrogen, the negative electrode should be in excess.
- Omitting the adjective “maintenance-free” and using only “VRLA batteries” is a trend in the battery world because monitoring is often overlooked by users.
- On the other hand, there should be no leakage of oxygen to achieve oxygen circulation. This is the second condition to achieve maintenance-free.
- The battery must be completely sealed (except for valves that open under overpressure). This allows for gas consumption and “internal recombination reactions” to balance during the overcharge phase and during float.
This places more demands on the structure and production of single cells and monolithic cells:
- — Stronger battery case
- —More precise manufacturing tolerances
- —The amount of electrolyte added
For example: VRLA batteries are considered to be more sensitive to environmental factors (temperature, ventilation). The above balance can easily be disrupted by high temperature and/or excessive charge/float voltage. Total cell/whole cell failure due to thermal runaway is a frequent occurrence.
Maintenance cost savings (water additions) are easily offset by increased monitoring costs. So, in fact, careful consideration must be given to whether experimenting with VRLA batteries is absolutely necessary. If saving space is important, a VRLA battery can be used as it can be installed anywhere (even upside-down in theory).
What happens inside the battery is invisible – although this is possible with conventional flooded batteries.
In order to reduce self-discharge and thus minimize fluid loss, lead-antimony alloys should be avoided and replaced with lead-calcium alloys with a calcium content of <0.1%. Calcium is used as a supporting skeleton in the positive and negative plates. The world also uses pure lead as a positive grid.
12V lead-acid battery installation
- Unpacking and inspection
Handling:
It is forbidden to apply force on the terminal part to prevent terminal damage and cracking of the sealing part;
Avoid battery inversion, drop or impact;
Absolutely avoid the use of metal wires such as steel ropes to prevent short circuit of the battery.
Check: appearance of packing box and battery – no damage;
Check: the number of batteries, accessories – all, all;
Refer to: instruction manual, installation drawing, precautions.
2. Precautions before installation
- After checking that there is no abnormality in the battery, install it in a designated place (such as a battery room);
- If the battery is placed in the battery room, it should be placed as low as possible in the battery room;
- Avoid installing batteries near heat sources such as transformers;
- Because the battery may generate flammable gas when it is stored, it should be installed away from devices that generate sparks (such as fuses);
- Before connecting, polish the battery terminals to make them appear metallic;
- Be careful with conductive material shorting battery positive and negative terminals.
- When multiple batteries are used together, first ensure that the batteries are connected correctly, and then connect the batteries to the charger or load. In this case, the positive terminal of the battery should be connected to the positive terminal of the charger or load, and the negative terminal to the negative terminal. If the battery is not connected to the charger correctly, the charger will be damaged, be careful not to connect it incorrectly. Remember to connect correctly.
- When wiring, pay attention to the connection is firm, but do not use too much force, so as not to damage the terminal, the recommended tightening torque is shown in Table 1. Do not use excessive force on the terminal, and each connecting nut and bolt must be tightened.
12V lead-acid battery maintenance and maintenance
Many people think that the battery is a very simple thing, and usually don’t pay much attention to maintenance. In fact, in the daily use of the car, the battery is also one of the most important components, and it should not be sloppy.
What should be paid attention to in the daily use of the battery? The reporter specially interviewed Zhou Yongjian, deputy general manager of Evergreen Storage Battery Co., Ltd., and Xu Jingxiong, general manager of Guangzhou Guangxiongsheng Industry and Trade Co., Ltd. Zhou Yongjian said that batteries are divided into starting batteries and traction batteries, and starting batteries include maintenance-free batteries and “water-filled” batteries. As far as cars are concerned, starting batteries are commonly used, because they can make the car store energy and then release it instantly, so with good quality starting batteries, the car starts more quickly.
Brand batteries are more secure.
Some issues that need to be paid attention to in the use and maintenance of 12V lead-acid batteries
- If the battery is not used for a long time, it will slowly discharge itself until it is scrapped. Therefore, the car should be started at regular intervals to charge the battery. Another method is to unplug the two electrodes on the battery. It should be noted that unplug the positive and negative electrode wires from the electrode column. First, unplug the negative wire, or remove the connection between the negative electrode and the chassis of the car. Then unplug the other end with the positive sign (+), the battery has a certain service life and will need to be replaced after a certain period of time. When replacing, the above sequence should also be followed, but when connecting the electrode wires, the sequence is exactly the opposite, first connect the positive electrode, and then connect the negative electrode.
- When the pointer of the ammeter shows that the storage capacity is insufficient, it should be charged in time. The charge level of the battery can be reflected on the instrument panel. Sometimes it is found on the road that the power is not enough, and the engine cannot be turned off. As a temporary measure, you can ask other vehicles for help, use the batteries on their vehicles to start the vehicle, connect the negative and negative electrodes of the two batteries, and connect the positive and positive electrodes. .
- The density of the electrolyte should be adjusted according to the standards in different regions and seasons.
- Distilled water or special rehydration should be added when the electrolyte is deficient. Do not use pure drinking water instead. Because pure water contains a variety of trace elements, it will cause adverse effects on the battery.
- When starting the car, the uninterrupted use of the starter will cause the battery to be damaged due to excessive discharge. The correct way to use it is that the total time to start the car each time is not more than 5 seconds, and the interval between restarting is not less than 15 seconds. In the case that the car does not start after several starts, the reason should be found from other aspects such as the circuit, the ignition coil or the oil circuit.
- Always check whether the holes on the battery cover are ventilated during daily driving. If the small holes of the battery cover are blocked, the generated hydrogen and oxygen cannot be discharged. When the electrolyte expands, the battery shell will be broken and the battery life will be affected.
- Check the positive and negative levels of the battery for signs of oxidation. You can often pour hot water on the battery’s wire connection, clean it with a copper wire brush, and apply butter.
- Check all parts of the circuit for aging or short-circuit. Prevents batteries from being retired early due to overdischarge.
- It is forbidden to store the battery without power. If it is used up for a few days and then recharged, the plate is prone to sulfation and the capacity decreases.
- Regular inspection: Regularly measure the voltage of a single battery. If the voltage of one of the batteries is lower than 10.5v, you should find a repair station for inspection or repair, so as not to damage the other two good batteries.
- The designed carrying capacity of the electric bicycle is 75kg. Avoid carrying heavy objects. Please use pedals to assist when starting and going uphill.
- It is normal for the battery capacity to decrease with the decrease in temperature in winter. Taking 20°C as the standard, the capacity is generally 80% at -10°C.
- Keep the surface of the battery clean for a long time. It is forbidden to expose to the sun when storing the vehicle. The vehicle should be parked in a cool, ventilated and dry place.
- When the battery needs to be placed for a long time, it must be fully charged first, and it is usually replenished once a month.
- The vehicle should be assisted by foot pedals when starting, going uphill, overloading, and in the wind to avoid high current discharge.
- Use a special charger when charging, and place it in a cool and ventilated place to avoid high temperature and humidity.
- Do not use organic solvents to clean the battery case.
- Do not short-circuit the positive and negative terminals of the battery to avoid danger.
- Over-discharge is prohibited: when the red under-voltage indicator on the instrument panel glows, it indicates that the power has entered the starvation area and should be charged in time.
- Overcharging is prohibited: The charging time should be different according to the mileage. The longer the mileage, the longer the charging time, and vice versa.
- If the battery pack fails, please send it to the authorized office of the manufacturer or relevant institutions for proper disposal. Please do not discard it at will to avoid environmental pollution.
How to activate 12V lead-acid battery
The commonly used batteries are idle for a long time, which is caused by serious vulcanization of the batteries. Check whether the batteries lack electrolyte, add distilled water, and do not cover and add liquid.
To activate, you need to find a 200-watt ordinary incandescent lamp and connect the battery in series, connect it to the 220V AC power supply at home, and see if the lamp can light up normally.
If it can light up normally, continue to power on for one minute, disconnect the power supply, wait a few minutes and connect it again, and repeat three to five times.
If the battery cannot be used for fifteen hours after being connected to the charger, you should choose a new battery.
Generally, the battery life is about two years. If you find that the car is difficult to start, please replace the battery as soon as possible.
12V alkaline battery
Alkaline batteries use manganese dioxide as the positive electrode, zinc as the negative electrode, and potassium hydroxide as the electrolyte. Its characteristics are better than that of carbon-zinc batteries, and its electric capacity is large. Taking gold-top alkaline batteries as an example, it has an average of 5 times more electricity than carbon-zinc batteries (power multiples will vary with different electrical products), good preservation, and leakage resistance. It has good liquid properties, good temperature resistance, small voltage change, stable voltage can be obtained, and high-efficiency discharge can still be achieved under high current, so both large and small current devices are suitable for use.
12V NiMH NiCd battery
Advantages and disadvantages of 12V NiMH batteries
As one of the new energy batteries, nickel-metal hydride batteries are highly concerned by the new energy battery industry, but whether they are lithium-ion batteries, fuel power cells or nickel-hydrogen batteries have their own advantages and disadvantages.
1. Advantages of NiMH batteries compared with lead-acid batteries and NiCd batteries:
(1) In terms of energy density, nickel-hydrogen batteries are larger than lead-acid batteries and nickel-cadmium batteries, and the battery capacity is large;
(2) In terms of discharge rate, that is, in terms of high current discharge, a rate discharge current of 15C can be achieved, but nickel-cadmium batteries and lead-acid batteries cannot;
(3) In terms of environmental protection, both lead-acid batteries and nickel-cadmium batteries pollute the environment and endanger the healthy development of animals and humans, while nickel-metal hydride batteries are relatively environmentally friendly and have little pollution to the environment;
(4) In terms of battery life, the service life of nickel-metal hydride batteries is longer than that of nickel-cadmium and lead-acid batteries;
(5) Nickel-cadmium batteries have a large memory effect, lead-acid batteries are easy to be vulcanized and passivated, and nickel-metal hydride batteries have a small memory effect;
2. Disadvantages of nickel-metal hydride batteries compared with lead-acid batteries and nickel-cadmium batteries:
(1) In terms of production and manufacturing costs, nickel-metal hydride batteries are relatively expensive;
(2) The self-discharge performance is relatively poor, that is to say, the self-discharge current is relatively large;
(3) Due to the high energy density, the safety performance is worse than that of nickel-cadmium batteries and lead-acid batteries;
3. Advantages of NiMH batteries compared to Li-ion batteries
(1) In terms of manufacturing cost and technology maturity, nickel-metal hydride batteries have lower cost and more mature technology;
(2) In terms of cell consistency, nickel-metal hydride batteries have better control and higher consistency than lithium-ion batteries;
(3) In terms of safety performance, due to the relatively low capacity density of nickel-metal hydride batteries, safety accidents of smoke and explosion are not easy to occur;
(4) In terms of raw materials, nickel-hydrogen batteries have more abundant resources, and lithium-ion batteries are relatively scarce;
4. Disadvantages of NiMH batteries compared with Li-ion batteries
(1) In terms of cycle life, nickel-metal hydride batteries are shorter than lithium-ion batteries;
(2) Ni-MH batteries are relatively low in energy density and relatively short in battery life;
(3) In high current discharge, that is, rate discharge, lithium-ion batteries can discharge above 45C, and nickel-metal hydride batteries can achieve about 15C;
(4) In terms of space utilization, nickel-metal hydride batteries are fixed cylindrical, and lithium-ion batteries can be soft-wrapped aluminum film, which can be diversified in shape and make full use of the space of the battery compartment of the product;
(5) In terms of low temperature performance, the discharge performance of nickel-hydrogen batteries is much worse than that of lithium-ion batteries;
(6) In terms of fast charging performance, nickel-hydrogen batteries are far worse than lithium-ion batteries. For example, lithium-ion batteries manufactured by battery production can achieve fast charging of 3 to 5C, while nickel-hydrogen batteries are better at 1C fast charging. That is to say, the charging time of NiMH batteries is much longer than that of Li-ion batteries.
The above is a brief overview of the advantages and disadvantages of nickel-metal hydride batteries made by battery technical engineers who are engaged in battery R&D and manufacturing. In fact, the comparison of the advantages and disadvantages of nickel-metal hydride batteries depends on what type of batteries they are compared with, rather than simply comparing their own properties.
Which one is more resistant to overcharge and overdischarge among the three types of batteries: nickel-cadmium battery, nickel-metal hydride battery and lithium battery?
Nickel-cadmium battery: The theoretical cycle times are more than 1000 times, the internal resistance is very small, and it can discharge up to 10C or more. Not afraid of overcharging and overdischarging, but it is extremely unbearable to charge without discharging. If the shallow discharge quality is not good, it can be reimbursed only 1-2 times. This type of battery has low self-discharge.
Nickel-metal hydride battery: The theoretical cycle times are more than 1000 times, the internal resistance is larger than that of nickel-cadmium, and it cannot discharge with high efficiency and high current. Compared with nickel-cadmium, overcharge has a larger heat release, but its endurance is much better than that of lithium battery. Over-discharge will damage the battery to a certain extent but it is acceptable. It only has a weak memory effect, so it can be charged without discharging. This type of battery has a large self-discharge.
Lithium battery: The theoretical cycle times are more than 300 times, the internal resistance is small, and it can discharge with high efficiency and high current, but unless it is a power battery, it cannot exceed 2C discharge. I am very afraid of overcharging, otherwise it will easily catch fire. Overdischarging will cause great damage to the battery, and there is basically no memory effect, so it can be charged without finishing. Lithium batteries are not very polluting the environment but can cause soil salinization (lithium hydroxide), because lithium ion activity is too strong (alkali metal) lithium batteries cannot work at high temperatures or they will explode and burn, but solid lithium batteries can work at a wider temperature than a safe temperature The range is still not as good as the previous two.
Nickel-cadmium battery (Nickel-cadmiumbattery, commonly referred to as NiCd, pronounced “nye-cad”) is a popular battery. This battery uses nickel hydroxide (NiOH) and metal cadmium (Cd) as chemicals to generate electricity.
Compared with other types of batteries, the advantages of nickel-cadmium batteries are: they can store a certain amount of energy with a small weight, high charging efficiency, little change in terminal voltage during discharge, small internal resistance, and low requirements for the charging environment.
The disadvantage of nickel-cadmium batteries is the memory effect and heavy metal pollution of cadmium.
It should be noted that the abbreviation of NiCad is the registered trademark of SAFTCorporaTIon, which should not be regarded as the abbreviation of general nickel-cadmium battery.
Nickel-metal hydride batteries (NiMH) are improved from nickel-cadmium batteries (NiCd). It provides higher capacity, less obvious memory effect, and lower environmental pollution (without cadmium-Cd) than nickel-cadmium batteries at the same price. It is called the most environmentally friendly battery. However, when compared with lithium-ion batteries, it has a relatively high memory effect and a higher self-discharge response. NiMH batteries are suitable for high power consumption products such as digital cameras, while for some devices that require high discharge rates, NiCd batteries are better.
Charge
When fast charging, the microcomputer in the charger can be used to avoid overcharging of the battery. Today’s nickel-metal hydride batteries contain a catalyst that can eliminate the danger caused by overcharging in time.
2H2+O2–catalyst–>2H2O
But this reaction is only valid for C÷10 hours from the time of overcharging (C=the capacity marked on the battery). When the charging process starts, the temperature of the battery will rise significantly, and some rapid chargers (less than 1 hour) have built-in fans to prevent the battery from overheating.
Some manufacturers believe that: using some simple constant current (and small current) chargers, regardless of whether there is a timer, can safely charge NiMH batteries, and the allowable long-term charging current is C/10h (the standard of the battery is C/10h). Divide the charge by 10 hours). In fact, some inexpensive wireless phone base stations and the cheapest battery chargers work this way. While this may be safe, it may have adverse effects on battery life. According to Panasonic’s “Ni-MH Battery Charging Guide” (link at the bottom of the page), long-term use of the trickle method (charging with a small current for a long time) may cause damage to the battery; to prevent damage to the battery, trickle The charging current should be limited between 0.033×C per hour to 0.05×C per hour, and the maximum charging time is 20 hours.
discharge
Care must also be taken during the use of the battery. For several batteries connected in series (such as the usual arrangement of 4 AA batteries in digital cameras), it is necessary to avoid the battery being completely drained, and then “reverse charging” (Reversecharging) occurs. This can cause irreparable damage to the battery. Often, however, these devices (such as the aforementioned digital cameras) are able to detect the discharge voltage of the batteries in series, and when it drops below a certain level, automatically shut down to protect the batteries.
A single battery will not have the above dangers, and will only discharge until the voltage is 0. This will not damage the battery, in fact, periodically discharging and then recharging it helps to maintain the capacity and quality of the battery.
NiMH batteries have a high self-discharge effect, about 30% or more per month. This is higher than the 20% monthly self-discharge rate of nickel-cadmium batteries. The fuller the battery is charged, the higher the self-discharge rate; when the charge drops to a certain level, the self-discharge rate will decrease slightly. The temperature at which the battery is stored has a significant effect on the self-discharge rate. Because of this, NiMH batteries that are not used for a long time are best charged to a “half full” state of 40%.
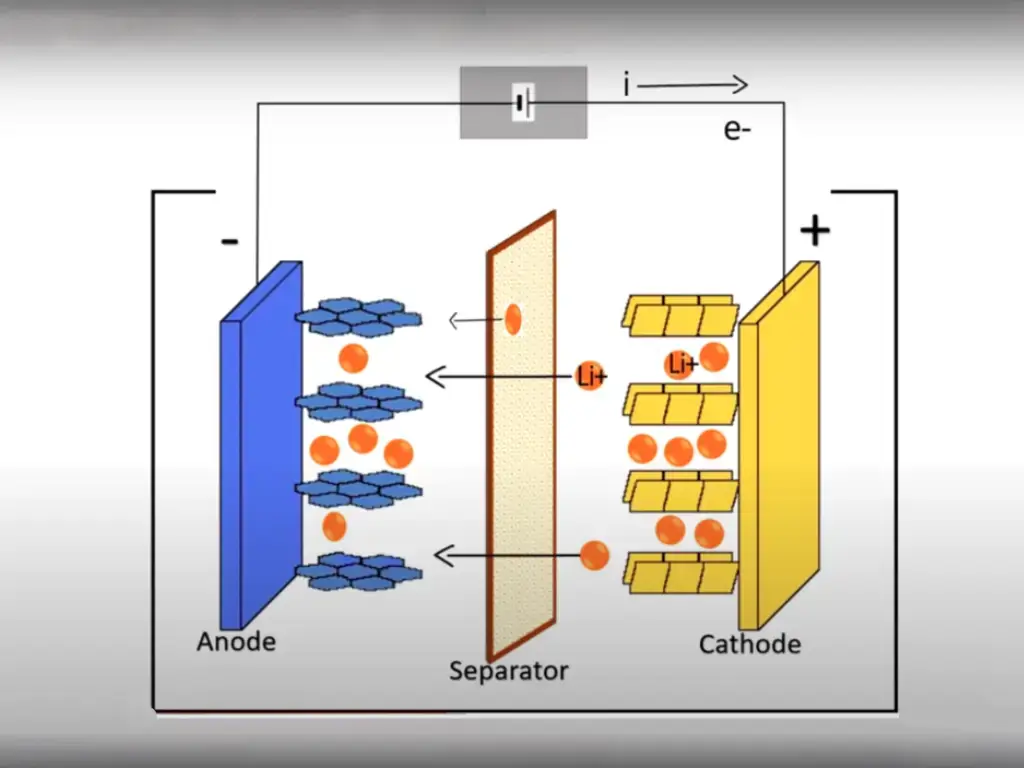
Lithium battery is a kind of battery that uses lithium metal or lithium alloy as negative electrode material and uses non-aqueous electrolyte solution. The inventor of the lithium battery was Edison.
Due to the very active chemical properties of lithium metal, the processing, storage and use of lithium metal have very high environmental requirements. Therefore, lithium batteries have not been used for a long time.
Lithium batteries generally have a nominal voltage higher than 3.0 volts and are more suitable for integrated circuit power supplies. Manganese dioxide batteries are widely used in computers, calculators, cameras, and watches.
The advantages and disadvantages of various lithium-ion batteries
The main development of global related companies is concentrated in “LiNiO2” (lithium nickel battery), “LiNi0.8Co0.2O2” (lithium nickel cobalt battery), “LiMn2O4” (lithium manganese battery), “” (lithium nickel cobalt manganese battery) and LFP (Lithium Iron Phosphate Battery). However, for power and energy storage batteries with medium and large capacity and medium and high power, the cost, discharge power, high temperature performance, and safety of cathode materials are very important, and not all of the above materials can meet these requirements.
The cost of lithium-nickel batteries is low and the capacity is high. However, the production process is difficult and the consistency and reproducibility of material properties are poor. The most serious thing is that there are still safety problems.
Li-Ni-Co battery is a solid solution (complex) of Li-Ni battery and Li-Co battery. It has the advantages of Li-Ni and Li-Co. It was once considered by the industry as a new cathode material most likely to replace Li-Co battery. The lifespan is poor, and there is still no further breakthrough in safety.
The cost of lithium manganese batteries is lower and the safety is much better than that of lithium cobalt, but the cycle life is not good, and the cycle life in high temperature environment is even worse. At high temperature, the phenomenon of manganese ion dissolution may even occur. The high temperature causes serious self-discharge, resulting in energy storage. Poor characteristics.
Lithium iron phosphate batteries have the main advantages of lithium cobalt, lithium nickel and lithium manganese at the same time, but do not contain precious elements such as cobalt, the price of raw materials is low, and phosphorus, lithium and iron are abundant in the earth’s resources, and there will be no supply problems. , Moreover, moderate operating voltage (3.2V), large capacity (170mAh/g), high discharge power, fast charging and long cycle life, high stability under high temperature and high heat environment, is currently considered by the industry as more suitable Lithium-ion batteries required by environmental protection, safety and high performance requirements.
However, the cathode material of lithium iron phosphate LFP batteries has always been subject to patent problems. At present, the three main technologies and compounds are mastered by three global manufacturers, including LiFePO4 from the University of Texas, and the other two Nanophosphate and NanoCocystallineOlivine (NCO ).
How to Choose the Best 12 Volt Battery Type For You
Picking the best 12-volt battery type for you is about trade-offs. Each battery type has advantages and disadvantages, and these can vary depending on your style of RV or travel.
The RVer on a tight budget may go for cheaper flooded lead-acid batteries, even if the long-term cost is more. Those who often operate in very hot or cold temperatures may want to avoid lead-acid batteries, however, in favor of a lithium-ion battery that will protect itself and perform better.
Gel batteries eliminate some of these issues, but the owner needs to be really comfortable with additional charging requirements.
RVers looking for low maintenance batteries should focus on sealed lead acid, gel, AGM, or lithium batteries and ignore flooded lead-acid batteries altogether.
Lithium-ion batteries are the obvious top choice, as they include an optimal mix of safety, low maintenance, efficiency, long lifespan, and power.
Darius Arberry LiFePO4
What is it that you need power for? keheng Lithium-Ion Batteries enable energy storage for thousands of people to live their dreams anywhere they go!