Lithium iron phosphate battery refers to a lithium ion battery using lithium iron phosphate as a positive electrode material. The cathode materials of lithium-ion batteries mainly include lithium cobalt oxide, lithium manganate, lithium nickel oxide, ternary materials, lithium iron phosphate, etc.
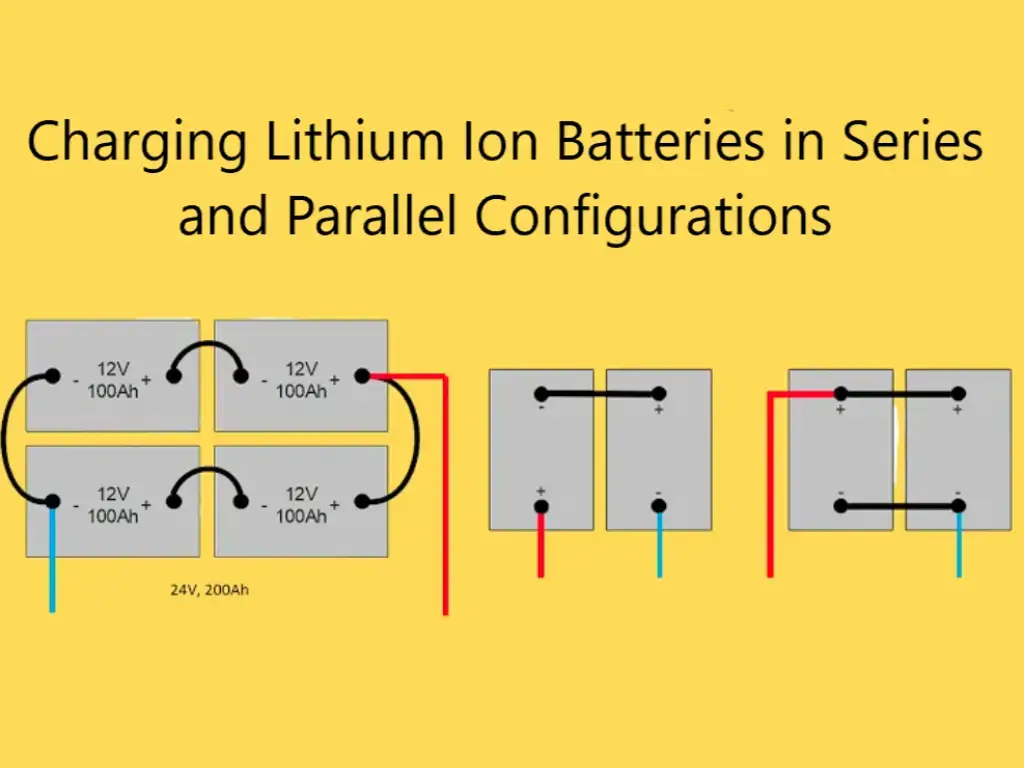
The positive electrode of lithium ion battery is lithium iron phosphate material, which has great advantages in safety performance and cycle life, which are also one of the most important technical indicators of power battery. The 1C charge-discharge cycle life can be achieved 2000 times, the puncture does not explode, and it is not easy to burn and explode when overcharged. Lithium iron phosphate cathode material makes large-capacity lithium-ion batteries easier to use in series.
Lithium iron phosphate battery refers to a lithium ion battery using lithium iron phosphate as a positive electrode material. The cathode materials of lithium-ion batteries mainly include lithium cobalt oxide, lithium manganate, lithium nickel oxide, ternary materials, lithium iron phosphate, etc. Among them, lithium cobalt oxide is the cathode material used in the vast majority of lithium-ion batteries. In terms of material principle, lithium iron phosphate is also an intercalation and deintercalation process, which is exactly the same as lithium cobaltate and lithium manganate.
Lithium iron phosphate battery is a lithium ion secondary battery, one of the main uses is for power batteries, which has great advantages over NI-MH and Ni-Cd batteries.
The charge and discharge efficiency of lithium iron phosphate batteries is high, and the charge and discharge efficiency can reach more than 90% under the rate discharge, while the lead-acid battery is about 80%.

9 advantages of lithium iron phosphate battery
Improvement of safety performance
The P-O bond in the lithium iron phosphate crystal is stable and difficult to decompose. Even at high temperature or overcharge, it will not collapse and generate heat like lithium cobalt oxide or form strong oxidizing substances, so it has good safety. A report pointed out that in the actual operation, a small number of samples were found to be burning in the acupuncture or short-circuit experiments, but no explosion occurred. In the overcharge experiment, a high voltage charging that was many times higher than the self-discharge voltage was used, and it was found that there were still explosion phenomenon. Even so, its overcharge safety has been greatly improved compared with ordinary liquid electrolyte lithium cobalt oxide batteries.
Improvement of lifespan
Lithium iron phosphate battery refers to a lithium ion battery that uses lithium iron phosphate as a positive electrode material.
The cycle life of long-life lead-acid batteries is about 300 times, and the maximum is 500 times, while the cycle life of lithium iron phosphate power batteries can reach more than 2,000 times, and the standard charging (5-hour rate) use can reach 2,000 times. The lead-acid battery of the same quality is “new half year, old half year, and maintenance and maintenance for half a year”, which is 1 to 1.5 years at most, while the theoretical life of lithium iron phosphate battery will reach 7 to 8 years when used under the same conditions. Comprehensive consideration, the performance-price ratio is theoretically more than 4 times that of lead-acid batteries. High-current discharge can quickly charge and discharge high-current 2C, under the special charger, the battery can be fully charged within 40 minutes of 1.5C charging, and the starting current can reach 2C, but lead-acid batteries do not have this performance.
Good high temperature performance
The electric heating peak of lithium iron phosphate battery can reach 350℃-500℃, while lithium manganate and lithium cobaltate are only around 200℃. The working temperature range is wide (-20C–+75C), and the electric heating peak of lithium iron phosphate with high temperature resistance can reach 350℃-500℃, while lithium manganate and lithium cobaltate are only around 200℃.
Large capacity
Has a larger capacity than ordinary batteries (lead acid, etc.). The monomer capacity is 5AH-1000AH.
No memory effect
Rechargeable batteries often work under the condition of being fully charged, and the capacity will quickly drop below the rated capacity. This phenomenon is called the memory effect. Like nickel-metal hydride and nickel-cadmium batteries, there is memory, but lithium iron phosphate batteries do not have this phenomenon. No matter what state the battery is in, it can be used at any time without having to discharge it before charging.
Lightweight
The volume of the lithium iron phosphate battery with the same specification and capacity is 2/3 of the volume of the lead-acid battery, and the weight is 1/3 of the lead-acid battery.
Environmentally friendly
The battery is generally considered to be free of any heavy metals and rare metals (nickel-metal hydride batteries require rare metals), non-toxic (SGS certified), non-polluting, in line with European RoHS regulations, and an absolute green battery certificate. Therefore, the reason why the lithium battery is favored by the industry is mainly due to environmental protection considerations. Therefore, the battery has been included in the “863” national high-tech development plan during the “Tenth Five-Year Plan” period, and has become a key project supported and encouraged by the state. With China’s entry into the WTO, the export volume of China’s electric bicycles will increase rapidly, and electric bicycles entering Europe and the United States have been required to be equipped with non-polluting batteries.
However, some experts said that the environmental pollution caused by lead-acid batteries mainly occurs in the non-standard production process and recycling process of enterprises. In the same way, lithium batteries belong to the new energy industry, but it cannot avoid the problem of heavy metal pollution. Lead, arsenic, cadmium, mercury, chromium, etc. in the processing of metal materials may be released into dust and water. The battery itself is a chemical substance, so it may cause two kinds of pollution: one is the process waste pollution in the production project; the other is the battery pollution after scrapping.
Lithium iron phosphate batteries also have their shortcomings: for example, low temperature performance is poor, the tap density of positive electrode materials is low, and the volume of lithium iron phosphate batteries of equal capacity is larger than that of lithium ion batteries such as lithium cobalt oxide, so it has no advantages in micro batteries. When used in power batteries, lithium iron phosphate batteries, like other batteries, need to face the problem of battery consistency.
Comparison of power batteries
At present, the most promising cathode materials for power lithium-ion batteries are mainly modified lithium manganate (LiMn2O4), lithium iron phosphate (LiFePO4) and nickel cobalt lithium manganate (Li(Ni,Co,Mn)O2) ternary Material. Nickel-cobalt lithium manganate ternary material is generally considered difficult to become the mainstream of power-type lithium-ion batteries for electric vehicles due to the lack of cobalt resources, high nickel and cobalt formation and large price fluctuations, but it can be combined with spinel manganic acid. Lithium is used in combination within a certain range.
Industry application
Carbon-coated aluminum foil brings technological innovation and industrial improvement to the lithium battery industry; improves the performance of lithium battery products and improves the discharge rate.
With the increasing requirements of domestic battery manufacturers for battery performance, new energy battery materials are generally recognized in China: conductive materials, conductive coated aluminum foil, and copper foil.
Its advantages are: when processing battery materials, it often has good high-rate charge-discharge performance and large specific capacity, but has poor cycle stability and serious attenuation, and has to make a choice.
It’s a magical coating that brings battery performance enhancements into a new era.
The conductive coating is composed of dispersed nano-conductive graphite-coated particles and the like. It provides excellent static conductivity and is a protective energy absorbing layer. It also provides good coverage protection. Coatings are water- and solvent-based and can be applied to aluminum, copper, stainless steel, aluminum and titanium bipolar plates.
Carbon coating brings the following improvements to the performance of lithium batteries:
Reduce the internal resistance of the battery and suppress the increase of the dynamic internal resistance during the charge-discharge cycle;
Significantly improve the consistency of the battery pack and reduce the cost of the battery pack;
Improve the adhesion between the active material and the current collector, and reduce the manufacturing cost of the pole piece;
Reduce polarization, improve rate performance, and reduce thermal effects;
Prevent the corrosion of the current collector by the electrolyte;
Comprehensive factor to extend battery life;
Coating thickness: conventional single-sided thickness 1 ~ 3μm.
In recent years, Japan and South Korea have mainly developed power lithium-ion batteries with modified lithium manganate and nickel-cobalt lithium manganate ternary materials as cathode materials, such as Panasonic EV Energy, a joint venture established by Toyota and Panasonic, Hitachi, Sony, New Kobe Electric, NEC, Sanyo Electric, Samsung and LG, etc.
The United States mainly develops power-type lithium-ion batteries with lithium iron phosphate as the positive electrode material, such as A123 Systems Company and Valence Company, but major American auto manufacturers choose manganese-based positive electrode material system power-type lithium-ion batteries in their PHEVs and EVs. And it is said that the American A123 company is considering entering the field of lithium manganate materials, while Germany and other European countries mainly adopt the way of cooperation with other countries’ battery companies to develop electric vehicles, such as Daimler Benz and the French Saft Alliance, Germany Volkswagen and Japan’s Sanyo Agreement cooperation Wait. At present, Volkswagen in Germany and Renault in France are also developing and producing power lithium-ion batteries with the support of their governments.
Disadvantages of lithium iron phosphate batteries
Whether a material has application development potential, in addition to focusing on its advantages, it is more critical whether the material has fundamental defects.
Domestically, lithium iron phosphate is generally chosen as the positive electrode material for power lithium-ion batteries. Market analysts from the government, scientific research institutions, enterprises, and even securities companies are optimistic about this material and regard it as the development direction of power lithium-ion batteries.
There are two main reasons to analyze the reasons: First, due to the influence of the research and development direction of the United States, the American Valence and A123 companies were the first to use lithium iron phosphate as the positive electrode material for lithium-ion batteries. Secondly, there is no lithium manganate material with good high temperature cycle and storage performance that can be used in power lithium-ion batteries in China. However, lithium iron phosphate also has fundamental defects that cannot be ignored, which can be summed up as follows:
- During the sintering process during the preparation of lithium iron phosphate, iron oxide may be reduced to elemental iron in a high-temperature reducing atmosphere. Elemental iron can cause micro-short circuit of the battery and is the most taboo substance in the battery. This is also the main reason why Japan has not used this material as a positive electrode material for power lithium-ion batteries;
- Lithium iron phosphate has some performance defects, such as low tap density and compaction density, resulting in low energy density of lithium-ion batteries. The low temperature performance is poor, and even nano-encapsulation and carbon coating did not solve this problem. When Dr. Don Hillebrand, director of the Energy Storage System Center of Argonne National Laboratory in the United States, talked about the low temperature performance of lithium iron phosphate batteries, he described it as terrible. The electric vehicle cannot be driven at low temperature (below 0°C). Although some manufacturers claim that the capacity retention rate of lithium iron phosphate batteries is not bad at low temperature, it is in the case of small discharge current and low discharge cut-off voltage. In this condition, the device cannot start working at all.
- The preparation cost of the material and the manufacturing cost of the battery are higher, the yield of the battery is low, and the consistency is poor. Although the nanoscale and carbon coating of lithium iron phosphate improves the electrochemical performance of the material, it also brings other problems, such as the reduction of energy density, the increase of synthesis cost, poor electrode processing performance, and harsh environmental requirements. Although the chemical elements Li, Fe and P in lithium iron phosphate are very rich and the cost is low, the cost of the prepared lithium iron phosphate product is not low. Even if the early research and development costs are removed, the process cost of the material is relatively high. The cost of preparing the battery will make the final unit energy storage cost higher.
- Poor product consistency. At present, there is no lithium iron phosphate material factory in China that can solve this problem. From the perspective of material preparation, the synthesis reaction of lithium iron phosphate is a complex heterogeneous reaction, including solid phase phosphate, iron oxide and lithium salt, plus carbon precursor and reducing gas phase. In this complex reaction process, it is difficult to ensure the consistency of the reaction.
- Intellectual property issues. The earliest patent application for lithium iron phosphate was obtained by F X MITTERMAIER & SOEHNE OHG (DE) on June 25, 1993, and the application results were announced on August 19 of the same year. The basic patent of lithium iron phosphate is owned by the University of Texas, and the carbon coating patent is applied by Canadians. These two basic patents cannot be bypassed. If the patent royalty is included in the cost, the product cost will be further increased.
In addition, judging from the experience in R&D and production of lithium-ion batteries, Japan is the first country to commercialize lithium-ion batteries and has been occupying the high-end lithium-ion battery market. Although the United States is leading in some basic research, so far there is no large-scale lithium-ion battery manufacturer. Therefore, it is more reasonable for Japan to choose lithium manganate as the positive electrode material for power lithium-ion batteries. Even in the United States, the number of manufacturers using lithium iron phosphate and lithium manganate as cathode materials for power lithium-ion batteries is equally divided, and the federal government supports the research and development of these two systems at the same time.
In view of the above problems of lithium iron phosphate, it is difficult to be widely used as a positive electrode material for power lithium-ion batteries in new energy vehicles and other fields. If the problems of high temperature cycling and poor storage performance of lithium manganate can be solved, with its advantages of low cost and high rate performance, the application in power lithium-ion batteries will have great potential.
- Working principle and characteristics The full name of lithium iron phosphate battery is lithium iron phosphate lithium ion battery, which is too long, and is referred to as lithium iron phosphate battery for short. Because its performance is particularly suitable for power applications, the word “power” is added to the name, that is, lithium iron phosphate power battery. It is also called “lithium iron (LiFe) power battery”.
In the metal trading market, cobalt (Co) is the most expensive and there is not much storage, nickel (Ni) and manganese (Mn) are cheaper, and iron (Fe) is the cheapest. The prices of cathode materials are also in line with those of these metals. Therefore, the lithium-ion battery made of LiFePO4 cathode material should be the cheapest. Another feature of it is that it does not pollute the environment.
As a rechargeable battery, the requirements are: high capacity, high output voltage, good charge and discharge cycle performance, stable output voltage, high current charge and discharge, electrochemical stability, safety in use (not due to overcharge, overdischarge and short circuit) It can cause combustion or explosion due to improper operation), wide operating temperature range, non-toxic or less toxic, and no pollution to the environment. The lithium iron phosphate battery using LiFePO4 as the positive electrode has good performance requirements, especially in terms of large discharge rate discharge (5-10C discharge), stable discharge voltage, safety (non-burning, non-exploding), life (cycle times) ), no pollution to the environment, it is the best, and is currently the best high-current output power battery.
The structure and working principle of lithium iron phosphate battery
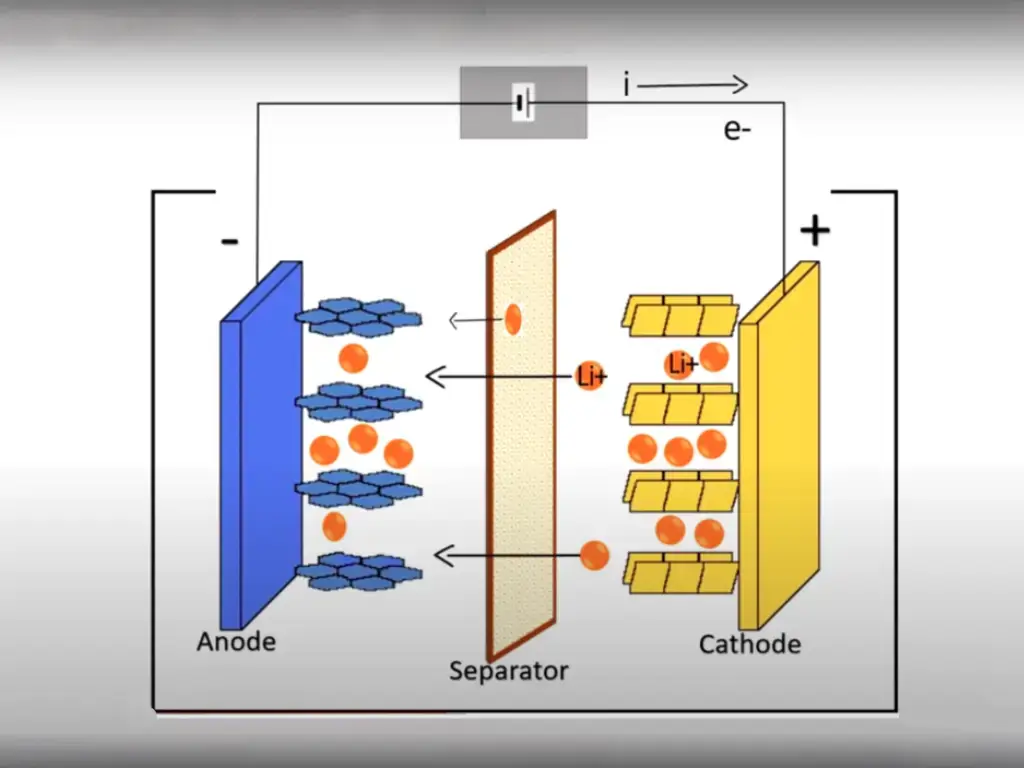
The internal junction of the LiFePO4 battery is LiFePO4 with an olivine structure as the positive electrode of the battery, which is connected to the positive electrode of the battery by an aluminum foil. , on the right is a battery negative electrode composed of carbon (graphite), which is connected to the battery negative electrode by a copper foil. Between the upper and lower ends of the battery is the electrolyte of the battery, and the battery is hermetically sealed by a metal casing.
When LiFePO4 batteries are charged, the lithium ions Li+ in the positive electrode migrate to the negative electrode through the polymer separator; during the discharge process, the lithium ions Li+ in the negative electrode migrate to the positive electrode through the separator. Lithium-ion batteries are named after lithium ions migrate back and forth during charging and discharging.
Main performance
The nominal voltage of the LiFePO4 battery is 3.2V, the final charge voltage is 3.6V, and the final discharge voltage is 2.0V. Due to the different quality and process of positive and negative electrode materials and electrolyte materials used by various manufacturers, there will be some differences in their performance. For example, the capacity of the battery of the same type (standard battery in the same package) is quite different (10% to 20%).
It should be noted here that lithium iron phosphate power batteries produced by different factories will have some differences in various performance parameters; in addition, some battery performance is not included, such as battery internal resistance, self-discharge rate, charge and discharge temperature, etc.
The capacity of lithium iron phosphate power batteries is quite different and can be divided into three categories: small tenths to a few milliamp hours, medium tens of milliamp hours, and large hundreds of milliamp hours. There are also some differences in the same parameters of different types of batteries. At present, the widely used small standard cylindrically packaged lithium iron phosphate power battery has a parameter profile size of 18mm in diameter and 650mm in height (model 18650).
Overdischarge to zero voltage test
The STL18650 (1100mAh) lithium iron phosphate power battery was used for the discharge to zero voltage test. Test conditions: 1100mAh STL18650 battery is fully charged with a 0.5C charge rate, and then discharged to a battery voltage of 0C with a 1.0C discharge rate. Then divide the batteries placed at 0V into two groups: one group is stored for 7 days, and the other group is stored for 30 days; after the storage expires, it is fully charged with a 0.5C charging rate, and then discharged with 1.0C. Finally, the differences between the two zero-voltage storage periods are compared.
The result of the test is that after 7 days of zero voltage storage, the battery has no leakage, good performance, and the capacity is 100%; after 30 days of storage, there is no leakage, good performance, and the capacity is 98%; after 30 days of storage, the battery is subjected to 3 charge-discharge cycles, The capacity is back to 100%.
This test shows that even if the battery is over-discharged (even to 0V) and stored for a certain period of time, the battery will not leak or be damaged. This is a feature that other types of lithium-ion batteries do not have.
Features of lithium iron phosphate battery
Through the above introduction, LiFePO4 battery can be summarized as the following characteristics.
High-efficiency output: standard discharge is 2~5C, continuous high current discharge can reach 10C, and instantaneous pulse discharge (10S) can reach 20C;
Good performance at high temperature: when the external temperature is 65 °C, the internal temperature is as high as 95 °C, and the temperature at the end of battery discharge can reach 160 °C, the structure of the battery is safe and intact;
Even if the battery is damaged internally or externally, the battery does not burn, does not explode, and has the best safety; excellent cycle life, after 500 cycles, its discharge capacity is still greater than 95%;
No damage even from over-discharge to zero volts; fast charging; low cost; no pollution to the environment.
Application of lithium iron phosphate power battery
Because lithium iron phosphate power batteries have the above characteristics, and produce batteries of various capacities, they are soon widely used. Its main application areas are:
Large electric vehicles: buses, electric vehicles, sightseeing vehicles and hybrid vehicles, etc.;
Light electric vehicles: electric bicycles, golf carts, small flat-panel battery vehicles, forklifts, cleaning vehicles, electric wheelchairs, etc.;
Power tools: electric drills, chainsaws, lawn mowers, etc.;
Remote control cars, boats, airplanes and other toys;
Energy storage equipment for solar and wind power generation;
UPS and emergency lights, warning lights and miner’s lights (the best safety);
Replace the 3V primary lithium battery and 9V nickel-cadmium or nickel-metal hydride rechargeable battery in the camera (the same size);
Small medical equipment and portable equipment, etc.
Here is an application example of replacing lead-acid batteries with lithium iron phosphate power batteries. Using 36V/10Ah (360Wh) lead-acid battery, its weight is 12kg, it can travel about 50km on a single charge, the number of charging times is about 100 times, and the use time is about 1 year. If the lithium iron phosphate power battery is used, the same 360Wh energy (composed of 12 10Ah batteries in series) is used, and its weight is about 4kg. It can travel about 80km once charged, the number of charging times can reach 1000 times, and the service life can reach 3 to 5 years. Although the price of lithium iron phosphate power battery is much higher than that of lead-acid battery, the overall economic effect is better to use lithium iron phosphate power battery, and it is lighter in use.
The performance of lithium-ion power batteries mainly depends on the positive and negative electrode materials. Lithium iron phosphate as a lithium battery material has only appeared in recent years. Large-capacity lithium iron phosphate batteries were developed in July 2005. Its safety performance and cycle life are unmatched by other materials, and these are the most important technical indicators of power batteries. 1C charge and discharge cycle life up to 2000 times. Single-cell battery overcharge voltage 30V will not burn, puncture will not explode. The lithium iron phosphate cathode material makes large-capacity lithium-ion batteries easier to use in series. In order to meet the needs of frequent charging and discharging of electric vehicles. It has the advantages of non-toxicity, non-polluting, good safety performance, wide source of raw materials, low price, long life, etc. It is an ideal cathode material for a new generation of lithium-ion batteries.
This project belongs to the development of functional energy materials in high-tech projects, and is the key support area of the national “863” plan, “973” plan and the “Eleventh Five-Year” high-tech industry development plan.
The positive electrode of lithium ion battery is lithium iron phosphate material, which has great advantages in safety performance and cycle life, which are also one of the most important technical indicators of power battery. The 1C charge-discharge cycle life can be achieved 2000 times, the puncture does not explode, and it is not easy to burn and explode when overcharged. Lithium iron phosphate cathode material makes large-capacity lithium-ion batteries easier to use in series.
Recently, there have been continuous reports about the progress of new batteries that are expected to replace traditional lithium batteries, giving us hope that mobile phones and tablets will have longer battery life, but unfortunately most of them remain in the laboratory research stage. Large-scale commercial use is hard to say.
In the white paper on lithium iron phosphate battery technology published by Deboch TEC.GmbH, after using composite nanomaterials, the energy density of a single cell of 32650 specification (diameter 32mm/length 65mm) can be increased to 6000mAh, which is comparable to the current industry 32650 specification single cell. Compared with the 5000mAh specification, the same volume has increased by a full 1000mAh, which is as much as 20%, and one cell can repeatedly charge the iPhone 4S mobile phone almost 4 times.
What is even more gratifying is that when used in a single low-rate charge-discharge environment, the battery remains at about 80% after being cycled for up to 3,000 times, while ordinary lithium batteries are cycled for about 500 times. . According to the calculation of charging and discharging every 3 days, it can be used continuously for 24 years, which is a veritable long-life battery.
This new battery technology can be widely used in portable power banks, small UPS, notebook batteries, car batteries and other equipment, and for different use environments, Deboch TEC.GmbH also uses different battery colors according to the number of cycles. : For the military grade, it is golden, and the number of cycles is 3000; the blue one is used in the field of civilian vehicles, 2500 times; the green one, 2000 times, is suitable for small portable mobile devices.