Introduction
Definition Of Lithium Battery Electrolyte
Lithium battery electrolyte refers to the conductive medium within a lithium-ion battery that allows for the movement of lithium ions between the positive and negative electrodes during charging and discharging cycles. It typically consists of a solvent, which provides a medium for ion transport, and a lithium salt, which enhances the electrolyte’s ionic conductivity. The composition and quality of the electrolyte play a crucial role in determining the performance, safety, and longevity of lithium batteries.
Electrolytes in lithium batteries are designed to be stable under various operating conditions while facilitating efficient ion transport. They must possess high ionic conductivity to allow rapid movement of lithium ions between electrodes during charge and discharge cycles.
Additionally, electrolytes must be chemically stable to prevent unwanted side reactions that could degrade battery performance or lead to safety hazards. The choice of electrolyte components and their proportions is critical in ensuring optimal battery performance.
On The Composition Of Lithium Battery Electrolyte
The composition of lithium battery electrolyte is of utmost importance in ensuring the battery’s efficiency and safety. It consists primarily of solvents and lithium salts.
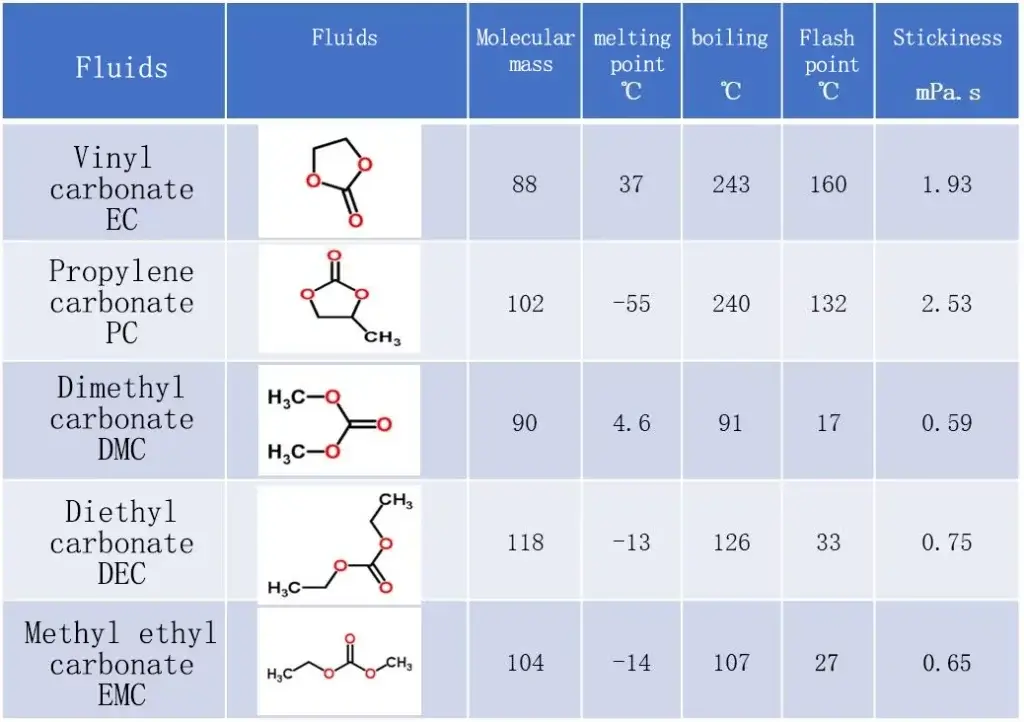
The selection of solvents for the electrolyte holds great significance in maintaining the conductivity and stability of the lithium battery. Among the commonly used solvents in electrolytes are ethylene carbonate (EC), dimethyl carbonate (DMC), and diethyl carbonate (DEC). These solvents aid in the dissolution of lithium salts and facilitate the required ionic conductivity for the battery.
Importance Of Electrolyte In Lithium Batteries
The electrolyte is a vital component of lithium battery as it directly influences their overall performance characteristics. It plays a key role in determining factors such as energy density, power output, cycle life, and safety features of the battery system.
A well-designed electrolyte formulation can significantly enhance the efficiency and reliability of lithium batteries for various applications ranging from portable electronics to electric vehicles. In addition to facilitating ion transport within the battery cell, the electrolyte also helps maintain proper electrode balance by enabling reversible electrochemical reactions during charging and discharging processes.
Moreover, advancements in electrolyte technology have paved the way for improved battery capacities, faster charging rates, enhanced thermal stability, and increased operational lifespan. Understanding the significance of electrolytes in lithium batteries is essential for optimizing battery design and performance across different industries.
Lithium-Ion And Lithium Polymer Batteries
Among the various types of lithium batteries, two predominant categories have emerged as industry standards – lithium-ion (Li-ion) and lithium polymer (LiPo) batteries. Lithium-ion batteries utilize a liquid electrolyte and are commonly found in numerous electronic devices such as smartphones, laptops, and electric vehicles.
They offer a high energy density and relatively low self-discharge rate, making them ideal for applications that require lightweight power sources with sustained performance. Lithium polymer batteries, on the other hand, feature a solid or gel-like electrolyte packaged in flexible pouch cells.
This design allows for more freedom in shaping and sizing the battery packs, making them popular choices for slim devices like wearables and drones. While initially slower to gain widespread adoption due to manufacturing challenges, lithium polymer batteries have gained traction for their enhanced safety features and versatility in design options.
The Composition Of Electrolyte In Lithium Batteries
Solvents: Ethylene Carbonate And Dimethyl Carbonate
The composition of the electrolyte in lithium batteries is a crucial aspect that directly impacts the performance and safety of the battery. Solvents play a key role in determining the electrolyte’s properties, such as viscosity and conductivity. Ethylene carbonate (EC) and dimethyl carbonate (DMC) are commonly used solvents in lithium battery electrolytes.
EC is known for its high dielectric constant, which helps in enhancing the conductivity of the electrolyte. On the other hand, DMC is valued for its low viscosity, which aids in efficient ion transport within the battery.
Lithium Salts: LiPF6 And LiBF4 For Enhanced Conductivity
In addition to solvents, lithium salts are essential components of lithium battery electrolytes that contribute significantly to enhancing conductivity. Among various lithium salts used, lithium hexafluorophosphate (LiPF6) and lithium tetrafluoroborate (LiBF4) are widely utilized for their ability to improve ionic conductivity within the battery. These salts dissociate into lithium cations and anions when dissolved in the solvent, facilitating ion movement between electrodes during charging and discharging processes.
The Synergistic Effect Of Solvents And Lithium Salts
The selection and combination of solvents with specific lithium salts have a synergistic effect on optimizing the overall performance of lithium batteries. By carefully choosing solvents with suitable properties like high dielectric constant or low viscosity, along with compatible lithium salts that enhance conductivity, manufacturers can tailor electrolytes to meet specific battery requirements regarding power output, cycle life, and safety. The precise balance between solvents and lithium salts is critical in achieving an efficient electrochemical reaction within the battery while ensuring stability under varying operating conditions.
The Critical Role Of Electrolyte In Lithium Batteries
Facilitating Ion Movement
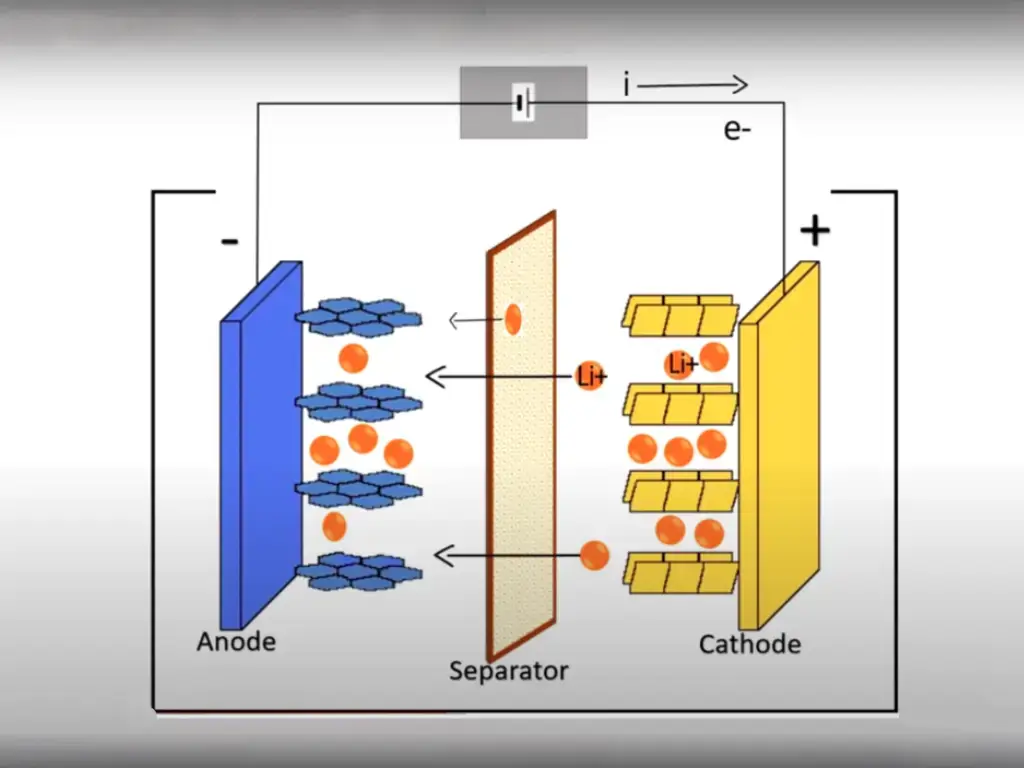
The electrolyte in lithium batteries plays a pivotal role in facilitating the movement of ions between the electrodes during both the charging and discharging processes. As the battery charges, lithium ions move from the positive electrode (cathode) to the negative electrode (anode) through the electrolyte. Conversely, during discharge, these ions travel back to the cathode.
This continuous flow of ions is essential for generating electrical current and powering various devices. The composition and properties of the electrolyte directly influence ion mobility and, consequently, battery performance.
Maintaining Performance And Longevity
In addition to enabling ion transport, the electrolyte also plays a crucial role in maintaining overall battery performance and longevity. The stability of the electrolyte affects factors such as cycle life, energy density, and rate capability – all of which are key indicators of battery quality.
A well-designed electrolyte can help mitigate issues like capacity fading and voltage instability that can occur over multiple charge-discharge cycles. By providing a stable environment for ion movement and electrode interactions, an effective electrolyte contributes to extending battery lifespan while sustaining optimal performance levels.
Optimizing Formulation For Enhanced Battery Efficiency
To ensure peak efficiency and longevity of lithium batteries, it is imperative to carefully consider various factors when formulating the electrolyte composition. From selecting appropriate solvents that offer high conductivity to choosing lithium salts that enhance ion transport efficiency, each component must be meticulously chosen to create an optimal blend.
Moreover, advancements in electrolyte technology continue to focus on improving thermal stability, reducing internal resistance, and enhancing safety measures within lithium batteries. By fine-tuning these formulations, researchers aim to unlock greater potentials for energy storage systems while prioritizing safety standards in evolving battery applications.
Unlocking The Secrets Of Electrolyte Conductivity
One of the critical factors influencing the performance of lithium battery electrolytes is conductivity, which dictates how easily ions can move within the electrolyte. Temperature plays a significant role in determining conductivity, as higher temperatures generally result in enhanced ion mobility due to increased thermal energy. However, extreme temperatures can also lead to degradation of the electrolyte components and reduce overall conductivity.
Concentration of lithium salts in the electrolyte solution also impacts conductivity, with higher concentrations typically resulting in greater ion mobility. It is essential for battery designers to carefully balance these factors to optimize overall performance and efficiency.
The Viscosity Conundrum: Balancing Mobility And Stability
Viscosity, or the resistance of a fluid to flow, is another crucial property that influences ion transport within lithium battery electrolytes. While low viscosity is desirable for facilitating rapid ion movement between electrodes during charging and discharging cycles, excessively low viscosity can lead to safety concerns such as leakage or dendrite formation within the battery.
On the other hand, high viscosity can impede ion transport efficiency and reduce overall battery performance. To achieve an optimal balance between mobility and stability, researchers are exploring various strategies such as incorporating additives or adjusting solvent compositions to fine-tune viscosity levels.
Additionally, advancements in nanostructured materials show promise in creating electrolytes with tailored viscoelastic properties that offer both efficient ion transport and enhanced safety features. By delving deeper into the complex interplay between viscosity and ion conduction, scientists aim to unlock new possibilities for next-generation lithium batteries with improved performance and longevity.
Safety Considerations With Lithium Battery Electrolytes
Risks Associated With Flammability And Chemical Reactivity
Lithium battery electrolytes can pose significant safety risks due to their flammability and chemical reactivity. The presence of flammable solvents in the electrolyte makes lithium batteries susceptible to thermal runaway and potential fires if not properly handled or designed.
Additionally, the chemical reactivity of some electrolyte components can lead to issues such as gas generation, which may cause pressure build-up within the battery cell and potentially result in rupture or explosion. These risks highlight the critical importance of ensuring strict safety measures during manufacturing, handling, and usage of lithium batteries.
Strategies For Enhancing Safety (E.G., Solid-State Electrolytes)
To mitigate the safety concerns associated with traditional liquid electrolytes, researchers have been exploring solid-state electrolytes as a safer alternative. Solid-state electrolytes offer improved stability and reduced flammability compared to liquid counterparts, thereby reducing the risk of fire incidents in lithium batteries.
By replacing volatile components with solid materials that exhibit high ionic conductivity, solid-state electrolytes provide a promising solution for enhancing battery safety without compromising performance. Implementation of solid-state electrolytes represents a crucial step towards ensuring the widespread adoption of lithium batteries across various applications.
Recent Advances In Electrolyte Technology
Solid-State Electrolytes As A Safer Alternative
Solid-state electrolytes have emerged as a cutting-edge solution to address safety concerns related to traditional liquid electrolytes in lithium batteries. These advanced materials offer enhanced stability, non-flammability, and improved resistance to thermal degradation, making them an attractive option for next-generation energy storage devices. Solid-state electrolytes also contribute to higher energy densities and better cycling performance while reducing the risks associated with internal short circuits or leakages common in liquid-based systems.
High Voltage Stable Electrolytes For Improved Performance
Innovations in high voltage stable electrolyte formulations have revolutionized the performance capabilities of lithium batteries by enabling operation at elevated voltages without compromising safety or cycle life. By optimizing the composition and properties of electrolyte materials, researchers have developed solutions that enhance electrochemical stability and support high-voltage operation over extended periods. This breakthrough not only boosts battery performance but also opens up new avenues for applications requiring increased energy density and efficiency.
Conclusion
Lithium battery electrolyte technology advancements signal a paradigm shift toward safer, more efficient energy storage solutions. Continued research and innovation are key to unlocking even greater potential in enhancing battery performance, safety, and sustainability. Embracing these developments paves the way toward a brighter, more sustainable future powered by cutting-edge energy technologies.
FAQ Of Battery Electrolytes
The composition of lithium battery electrolyte includes solvents and lithium salts, which are essential for the battery’s performance and safety.
Common solvents used in lithium battery electrolytes include ethylene carbonate (EC), dimethyl carbonate (DMC), and diethyl carbonate (DEC).
Commonly used lithium salts in electrolytes are lithium hexafluorophosphate (LiPF6), lithium perchlorate (LiClO4), and lithium tetrafluoroborate (LiBF4).
The electrolyte facilitates the movement of lithium ions between the anode and cathode, enabling the flow of electrical current and impacting the battery’s performance and efficiency.
An ideal electrolyte for lithium batteries should have low viscosity, high conductivity, and stability at high voltages to ensure efficient ion transport, reliable power output, and longevity of the battery.
The primary challenges and safety concerns with lithium battery electrolyte include flammability and thermal instability, which can lead to fires, explosions, and other safety hazards.
Recent developments in lithium battery electrolyte technology include the use of solid-state electrolytes and additives to enhance safety, stability, and performance of lithium batteries.
The electrolyte composition can impact the lifespan of lithium batteries by influencing degradation mechanisms, such as capacity fade and increased internal resistance. Optimizing the electrolyte composition can help extend the battery’s longevity.
Environmental and regulatory considerations for lithium battery electrolyte include recycling and disposal methods, as well as compliance with standards and guidelines to minimize environmental impact and ensure safe handling.