
Introduction
With tech zooming ahead, lithium batteries are powering up just about everything. From our phones to our electric rides, they’re everywhere. But ever paused to think about how are lithium batteries made? Let’s dive into the world of lithium batteries and unpack the smarts and science behind them.
What is a Lithium Battery?
A lithium battery is like a rechargeable power pack. This rechargeable battery uses lithium ions to pump out energy. No wonder they’re often called the MVPs of energy storage. Take regular batteries, for example, which can store around 100-200 watt-hours per kilogram (Wh/kg) of energy. But lithium ones? They can pack a massive 250-670 Wh/kg. Impressive, huh? The way they work is kinda like a dance: lithium ions shuffle from one end to the other, creating electricity. And when you plug them in to charge? Those ions shuffle right back to their starting point. No shocker they’re the go-to for our phones, laptops, and even electric rides.
Lithium batteries come in all shapes and flavors. There’s the fan-favorite lithium-ion, the flexible lithium-polymer, and the rugged lithium iron phosphate. Each has its own special thing going on. And they’re not just for the small stuff. The rise of electric cars shows just how game-changing these batteries are. It’s not only about their strong chemistry and handy benefits; they’re the driving force behind some of the coolest energy innovations today.
You May Like: AGM Battery vs Lithium Showdown: Which Powers Your Needs Best?
The Core Components of Lithium Battery
Ever stopped to think about what’s buzzing inside these little energy beasts? There’s a whole world of parts in the li-ion batteries, all teaming up to store and dish out energy like pros. The real magic of a lithium battery isn’t just its kick; it’s the harmony of all its bits and pieces jamming together. So, let’s dive in and get up close and personal with the nuts and bolts that make these batteries rock.
The Electrode
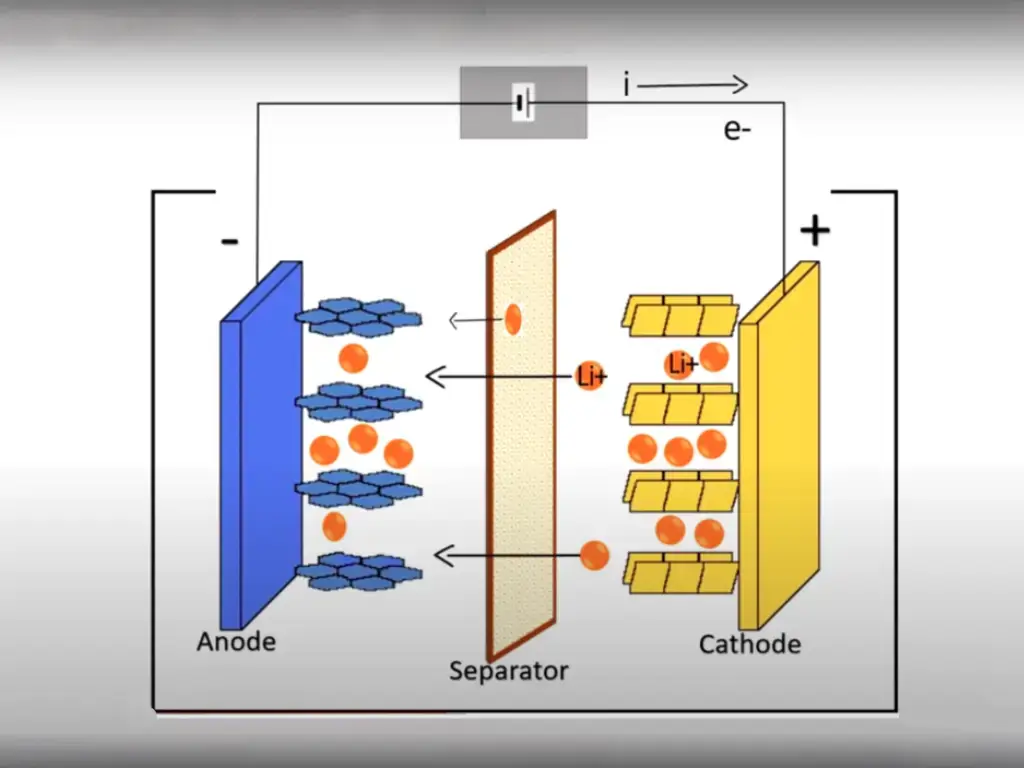
At the heart of a lithium battery, you’ve got the electrodes: the anode and cathode. Think of them as the DJs controlling the electron beats. The anode often rocks with metals that are into oxidizing, like graphite or zinc. Take graphite—it can stash up to 372 mAh/g, which is huge because that’s how we measure the battery’s energy stash. On the flip side, the cathode might jam with stuff like lithium cobalt oxide, known for its energy-packed vibes, or lithium iron phosphate, which is all about keeping things steady.
When you’re juicing up, lithium ions groove over to the anode. But when you’re jamming on your device, these ions slide over to the cathode, dropping the energy beats that power up your tech. This groovy ion shuffle, all thanks to the rad choice of electrode materials, ensures your battery’s got the moves and keeps the party going for a long time.
Separator
The separator’s like the quiet referee between the anode and cathode, making sure they stay in their corners. Why? ‘Cause if they got too chummy, you’d get a short circuit, and that’s a no-go for the battery’s vibe and safety. But this separator isn’t just standing there; it’s got these tiny gateways that let lithium ions dart through, ensuring the energy keeps on rocking. For the design geeks out there, it’s this killer combo of defense and flow that makes the separator the unsung hero in crafting batteries that are both beastly and safe.
Electrolyte
Often an overlooked detail in battery discussions, the electrolyte serves as the bustling highway for lithium ions, guiding them between the anode and cathode. Unlike a mere filler, it’s typically composed of lithium salts in an organic solvent, or it could be a solid polymer.
The choice of electrolyte can significantly influence your battery’s performance metrics—be it energy density, charge cycles, or temperature tolerance. For example, lithium hexafluorophosphate in organic solvents is renowned for its superior ionic conductivity, a pivotal factor that boosts the battery’s efficiency. In contrast, solid polymer electrolytes are gaining attention for their safety features, albeit at a slight compromise on energy density.
The electrolyte is far more than just a facilitator; it’s an active determinant of a battery’s reliability and efficiency. Tailoring the electrolyte to suit your application can make or break the overall performance.
Current collectors
Ever considered what fuels our phones and electric rides? Bingo, it’s the humble lithium battery. These compact power buddies are central to the modern gadgetry, ensuring we’re not perennially on a charger hunt. But what’s the saga of bringing these crucial bits to life? In this article, we’ll unravel the complex dance of how lithium batteries come to be, taking you from the raw materials to the cool gadget in your hand. Ready for a captivating trek? Let’s get rolling! Consider current collectors as the unsung maestros of batteries. They’re slim sheets, one of copper and the other of aluminum. Their big gig is to distribute electricity evenly within the battery.
Why the fuss? If electricity gangs up in one corner, it could spell trouble for the battery, even making it unsafe. For instance, uneven electric current can lead to lithium clumping together, triggering short circuits, which is a dicey scenario. Current collectors ensure your battery stays in the groove and out of trouble.
But wait, there’s more. Current collectors also jazz up the battery’s performance by ensuring the electric flow stays steady. This is crucial for things like electric cars and medical gear that need the reliability badge.
The choice of materials ain’t random either. Copper and aluminum are the stars of the show. Copper, with its stellar electric-conducting chops, is perfect for one side of the battery. Aluminum, being light and a good conductor, rocks the other side.
So, while the chatter often veers towards other battery parts like electrodes or electrolytes, let’s not forget about the current collectors. They’re the unsung heroes ensuring everything hums along nicely and safely.
Battery Casing
Battery shells, made from tough metals or top-notch plastics, keeping the precious insides safe from baddies like falls or wild weather.
Besides playing bodyguard, these shells play a huge part in keeping the battery’s temp just right, ensuring it’s always in the sweet spot for best performance. This dual role of playing protector and thermostat is key to making sure the battery lives a long and happy life.
Getting how important the battery shell is? That’s where KH Litech steps in, offering tailor-made lithium battery solutions. They’ve got a ton of ways to tweak things – from resizing the shell, changing its look, to even adding your very own logo and packaging. So, it’s not just about the function, but also making a statement. In short, the shell isn’t just a bodyguard; it’s also a canvas for creativity and brand vibes.
The Importance of BMS in Ensuring Safety
The Battery Management System (BMS) is like the brain of a lithium battery. It’s the boss, watching over things like charge level, temperature, and voltage. For example, if it sees a cell’s voltage dipping too low—say below 2.5V—it’ll step in and stop any more discharge to avoid damage.
But the BMS isn’t just playing defense; it’s also boosting the battery’s game. By keeping everything within safe levels, it adds a ton to the battery’s lifespan. With a well-tuned BMS, you can see up to a 5% improvement in state of charge accuracy, which means more months or even years of life for the battery.
Here at KH Litech, we get how crucial a solid BMS is for the battery’s safety and longevity. That’s why we pack our batteries with top-notch components, including lithium iron phosphate cathodes and a rock-solid BMS. It promises longer life, safety, and sharp state of charge calculations. Plus, our batteries are armed with a bunch of protections against overcharging, over-discharging, overcurrent, shorts, and overheating.
The BMS isn’t just a watchdog; it’s a game changer for lithium battery tech. Choosing KH Litech isn’t just picking up a battery—it’s investing in a secure and enduring power source.
Each piece of this tech plays its own part but works together like a symphony. And while we might not think about it when we’re using our gadgets, the brilliant engineering and design behind these batteries are something to admire. From the electrodes to the BMS, it’s all working in harmony to keep our tech-filled lives running smoothly and full of juice.
How are lithium ion batteries made?
The creation of lithium-ion batteries is a meticulous ballet of science and engineering, where every step is executed with unparalleled precision.
Electrodes Manufacturing
Making the electrodes is where the battery’s journey begins. They’re like the heart of a battery. First, we use raw materials, mainly graphite for the anode and different lithium compounds for the cathode, and we clean them up real good. This step is crucial because any dirt or impurity can mess up the battery’s performance.
After they’re all cleaned up, we mix these materials with special solvents to make a smooth slurry. Getting the right consistency is super important; even small changes can mess with how much energy the battery can store and how fast in charging or discharging process. Next, we spread this slurry onto metal foils—copper for the anode and aluminum for the cathode—in a step we call “coating.” The coating needs to be even, or it can create hotspots and shorten the battery’s life.
After coating, the foils go through high-precision drying tunnels to get rid of the solvent, leaving only the electrode material behind. When they’re dry, we cut these electrode sheets into exact shapes and sizes, custom-made to fit the battery we’re building.
In a nutshell, making electrodes is a mix of art and science, where every detail matters, and precision is key. It sets the stage for building a battery that’s strong and dependable.
Battery Cell Manufacturing
Diving deeper into how lithium batteries are made, we hit the action-packed assembly line. This is where all the bits and pieces come together to form our energy beast. Precision and know-how rule the day here.
First up, we prep those electrode sheets. The anode and cathode sheets get a special coating of active materials that helps them store and release energy. Then comes the separator—it’s a thin but tough little layer that sits between the sheets. It keeps them from touching each other but lets those ions zip through without a hitch.
After that, it’s time for the electrolyte. This isn’t just any liquid; it helps those lithium ions move around and also acts like a conductor to boost the battery’s performance. All these components then get a cozy home—either a strong metal tube or a flexible pouch, depending on what the job calls for. In the next step, the assembled cell structure is connected to the terminals or cell tabs, together with any safety devices, using an ultrasonic or laser welding process.
But we’re not done yet. Every single battery cell gets put through its paces with some intense testing. We’re talking checking things like voltage levels and how they handle heat, all to make sure they’re top-notch and super safe.
From Individual Cells to Battery Packs
Ever wonder how the tiny li-ion cells in your smartphone battery end up powering the device for hours? These individual cells are grouped into battery packs.
Imagine a single cell as a solo musician. Powerful on its own, but when combined with others, it forms an orchestra. In the realm of electric vehicles (EVs), this orchestra can consist of thousands of cells. For instance, a typical EV battery pack might contain 4,000 cells, delivering a combined voltage of around 400V. These cells can be connected in series to increase voltage or in parallel to boost capacity.
The building process is super detailed. Every cell is carefully checked for voltage uniformity to make sure they all perform the same. Then, they’re sorted by their capacities and volts, a crucial step to avoid any hiccups when they’re working. Once sorted, they’re linked up with busbars and overseen by a central Battery Management System (BMS) for peak performance.
But it’s more than just linking cells together. Systems to manage heat are put in place to keep temperatures in check, making sure the pack doesn’t get too hot. Protective shells are thrown on to guard the setup from any outside dangers.
Turning individual cells into a battery pack is like creating a work of art. It’s a mix of accuracy, engineering, and creativity, making sure whether it’s a phone or an EV, it gets a dependable, effective, and enduring source of power. And all this, while adhering to industry standard practices and optimizing power density, ensuring that every piece is a masterpiece in electrochemistry, far removed from the world of internal combustion engines.
Quality Control in Lithium Battery Manufacturing
In the lithium battery world, quality isn’t just about how well it works—it’s about keeping things safe. Using them the wrong way can be risky, but a battery made without top-notch checks? That’s like a hidden danger waiting to pop. Picture this: a battery with a tiny off-kilter part inside might seem no biggie at first. But as time goes on, this little hiccup can cause uneven wear, maybe even short circuits, or, in a worst-case scenario, spark a fire.
Enter KH Litech’s rigorous quality control regimen. Every battery emerging from our production line is subjected to a battery of tests, both visual and performance-based. For instance, a capacity test might reveal if a battery delivers 4900mAh instead of the promised 5000mAh, ensuring no consumer gets short-changed. Similarly, thermal tests ensure that even under extreme conditions, our batteries remain cool and composed.
Our commitment to quality doesn’t stop at testing. We meticulously select each cell and accessory, adhering strictly to ISO manufacturing standards. Our choice of lithium iron phosphate as a core material is deliberate—it’s renowned for its safety attributes, eliminating risks of fires or explosions. And, reinforcing this safety net is our integrated BMS, acting as a vigilant guardian, ensuring each battery operates within its safe parameters.
Comprehensive Testing of Lithium Batteries Prior to Market Introduction
For folks designing and building electronic gadgets, making sure lithium batteries are safe is a big deal. How reliable and safe a battery is can make or break a product.
Before a lithium battery gets the green light to leave the factory, it goes through a bunch of tough tests. Here’s the lowdown on what happens:
| Eye Check | First up, each battery gets a good look-over to make sure there’s nothing wonky or out of place. |
| Electricity Tests | These tests check out things like how much juice the battery can hold, its internal resistance, voltage, and other important stuff to make sure everything’s up to snuff. |
| Rough Handling | We put batteries through the wringer with shakes, shocks, and drops. This is to see how they’d handle being shipped, installed, or if someone’s a bit clumsy. |
| Hot and Cold Tests | We put batteries in super cold and super hot conditions to see how they’d do in all kinds of weather. |
| Pushing the Limits | We intentionally mess with the battery by causing a short circuit or overcharging it. This is to make sure the battery’s safety features jump in when things go south. |
| Life Cycle Tests | Here, we charge and discharge batteries over and over to see how long they’ll last and how well they perform over time. |
So, in a nutshell, we make sure every battery is put through the gauntlet before it hits the shelves. For the tech pros out there, knowing the ins and outs of these tests means peace of mind. It’s all about building trust between the folks making batteries and the people using them.
Also Read: How to fix a dead lithium ion battery?
Recycling and Repurposing: Breathing New Life into Spent Lithium Batteries
Lithium batteries don’t just call it quits when they can’t charge up anymore. As the world’s craving for these batteries keeps growing, we’ve got to think about what happens after they’ve done their time. It’s not just about making them anymore; it’s about making sure they don’t mess up our planet when they’re done.
Check this out: inside every lithium-ion battery, you’ve got some pretty valuable stuff like lithium, cobalt, and nickel. Tossing them means we’re throwing away these goodies and, worse, risking messing up the environment. Take cobalt, for example. Digging it up isn’t easy on Mother Earth. But if we recycle, we can get back up to 95% of this metal from an old battery, cutting down the need to mine more.
But there’s more than just recycling. We’re also looking at giving these batteries a second shot at life. Maybe a battery’s not up for powering a car anymore, but it’s still got some juice left for simpler jobs, like grid-scale energy storage or being a backup power source. This way, we’re squeezing every last drop of life out of it before it hits the trash.
Plus, some smart folks out there are figuring out how to give old batteries a makeover, swapping out the worn-out parts to make them feel brand new. This not only saves resources but also gives folks a cheaper option.
So, the story of lithium batteries is getting a plot twist. It’s not just a straight line from making them to tossing them. It’s more like a circle of life—use, reuse, and recycle, making sure we get the most bang for our buck. And as folks who care about our planet and love cool tech, it’s on us to make sure this cycle keeps spinning.
Safety Precautions in Lithium Battery Manufacturing
Safety is the name of the game when we’re talking about making lithium batteries. From start to finish, every step is all about keeping things safe and sound—for both the battery and the folks making them.
Starting with digging up the raw stuff, we gotta make sure it’s top-notch quality. Even the tiniest bit of unwanted stuff can mess with the battery’s mojo or even make it unsafe. Like, when we’re pulling out lithium, we aim for a purity of 99.5% or even better. This isn’t just about making the battery work great; it’s about keeping things on the safe side.
Jumping to the assembly line, safety gets dialed up to eleven. Good airflow? Absolutely essential. Some steps, like when we add the electrolyte, can let off some fumes. Without fresh air, these fumes can be bad news for workers and can even be a fire risk.
And you bet everyone’s decked out in safety gear. Think gloves, safety glasses, and even special suits to keep any chemicals or sparks at bay. Keeping an eye on the equipment is a must-do, too. Making sure things like temperature stay just right during the pressing process stops things from getting too hot and risky.
With all the techy advances, our safety game is even stronger. Modern factories have sensors everywhere, checking on stuff like room temperature, moisture, and fume levels. If something’s not right, alarms go off, and we jump into action.
Making lithium batteries isn’t just about giving them juice. It’s about doing it the right way, where safety and quality go hand in hand. Every battery that rolls out is a testament to a process that’s got everyone’s back.
Conclusion
Closing out our chat on lithium batteries, it’s a no-brainer they’re not just cool—they’re total game-changers. As we push for a greener tomorrow and ride the renewable energy wave, these batteries are taking the lead. And the best part? They’re upping their game, lasting longer, and turning even more eco-friendly. Peeling back the curtain on their making, we spot the killer mix of science and tech that’s powering our day-to-day. Whether it’s the phone you’re taking selfies with or the electric ride you’re cruising in, the future’s all kinds of bright—all thanks to the lithium buzz.



2 thoughts on “How Are Lithium Batteries Made? A Comprehensive Guide”
li-ion and li- polymer which battery is more heating for play games like pubg
Hi Doreen Gano
have a good day!
When playing intense games such as PUBG on mobile devices, the heat generated by batteries can be a concern. Lithium-ion (Li-ion) and lithium-polymer (Li-polymer) batteries are commonly used in portable electronic devices, including smartphones and gaming devices. Battery heat during gaming depends on a number of factors, including the chemistry of the battery, its design, and the way the device manages power.
In general, both Li-Ion and Li-Polymer batteries heat up when playing intense games such as PUBG. The degree of heat generation depends more on the quality of the battery, the design of the device, and the intensity of the game than just the type of battery. However, Li-Polymer batteries may be slightly better at generating heat because they have lower internal resistance and can be better integrated into the device to improve heat dissipation.
Sincerely
CT!