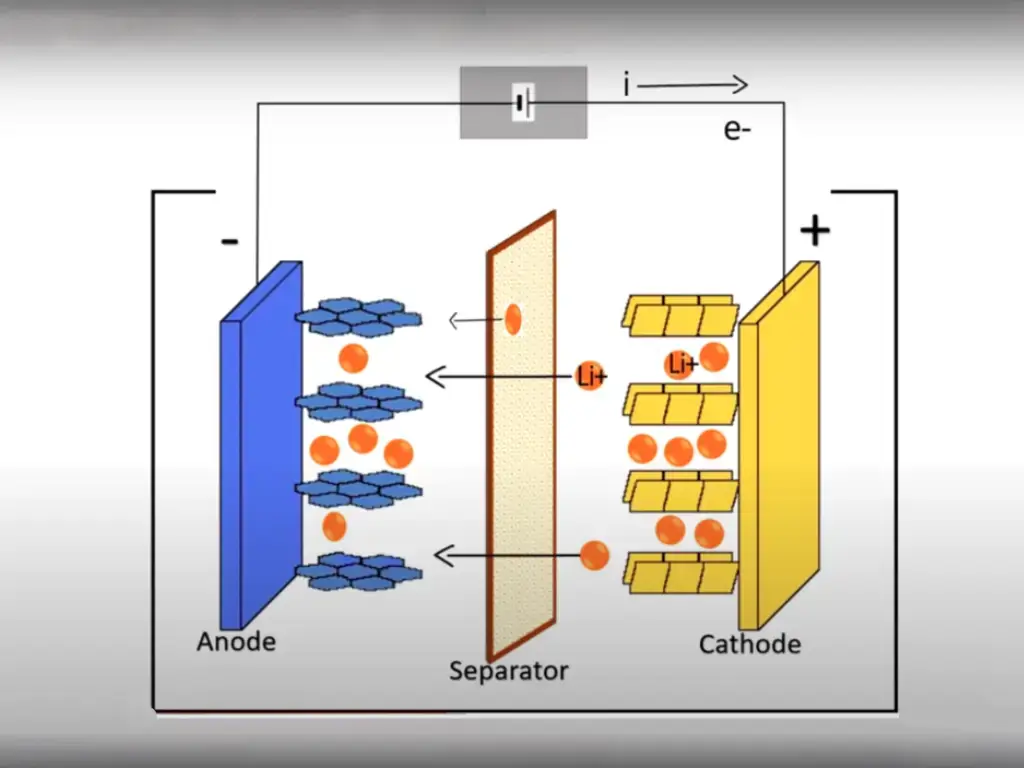
The cathode material is a key part of lithium-ion batteries and must meet the requirements of high capacity, strong stability and low toxicity.
Compared with other cathode materials, LiFePO4 electrode materials have many advantages, such as higher theoretical specific capacity, stable working voltage, stable structure, good cyclability, low cost of raw materials and environmental friendliness.
Therefore, LiFePO4 is an ideal cathode material and is selected as one of the main cathode materials for power batteries.
Many researchers have studied the mechanism of the accelerated performance degradation of LIBs at low temperature, and it is believed that the deposition of active lithium and its catalytically grown solid-state electrolyte interface (SEI) lead to the decrease of ionic conductivity and the decrease of electron mobility in the electrolyte. drop, which leads to a reduction in the capacity and power of LIBs and sometimes even battery performance failures.
The low temperature working environment of LIBs mainly occurs in winter and high latitude and high altitude areas, where the low temperature environment will affect the performance and life of LIBs, and even cause extremely serious safety problems. Affected by the low temperature, the rate of lithium intercalation in graphite is reduced, and metal lithium is easily precipitated on the surface of the negative electrode to form lithium dendrites, which pierce the diaphragm and cause an internal short circuit in the battery.
Therefore, methods to improve the low-temperature performance of LIBs are of great significance for promoting the use of electric vehicles in alpine regions.
This paper summarizes the methods to improve the low temperature performance of LiFePO4 batteries from the following four aspects
1) Pulse current generates heat;
2) Use electrolyte additives to prepare high-quality SEI films;
3) Interface conductivity of surface coating modified LiFePO4 material;
4) Bulk conductivity of ion-doped modified LiFePO4 material.
Pulse current heat generation
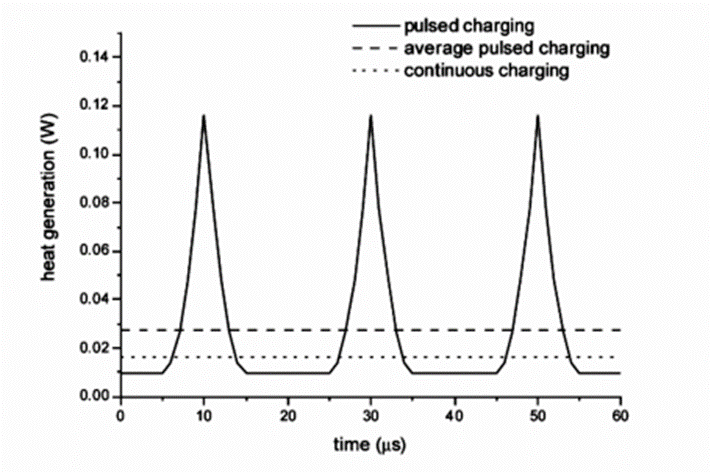
During the charging process of LIBs rapidly heated by pulse current, the movement and polarization of ions in the electrolyte will promote the internal heat generation of LIBs. This heat generation mechanism can be effectively used to improve the performance of LIBs at low temperature. Pulse current refers to a current whose direction does not change and whose current intensity or voltage changes periodically with time. To rapidly and safely increase the battery temperature at low temperatures, how the pulsed current heats the LIBs is theoretically simulated, and the simulation results are verified by experimental tests on commercial LIBs. The difference in heat generation between continuous charging and pulsed charging is shown in Figure 1. As can be seen from Figure 1, the microsecond pulse time can promote more heat generation in the lithium battery.
The cathode material is a key part of lithium-ion batteries and must meet the requirements of high capacity, strong stability and low toxicity.
Compared with other cathode materials, LiFePO4 electrode materials have many advantages, such as higher theoretical specific capacity, stable working voltage, stable structure, good cyclability, low cost of raw materials and environmental friendliness.
Therefore, LiFePO4 is an ideal cathode material and is selected as one of the main cathode materials for power batteries.
Many researchers have studied the mechanism of the accelerated performance degradation of LIBs at low temperature, and it is believed that the deposition of active lithium and its catalytically grown solid-state electrolyte interface (SEI) lead to the decrease of ionic conductivity and the decrease of electron mobility in the electrolyte. drop, which leads to a reduction in the capacity and power of LIBs and sometimes even battery performance failures.
The low temperature working environment of LIBs mainly occurs in winter and high latitude and high altitude areas, where the low temperature environment will affect the performance and life of LIBs, and even cause extremely serious safety problems. Affected by the low temperature, the rate of lithium intercalation in graphite is reduced, and metal lithium is easily precipitated on the surface of the negative electrode to form lithium dendrites, which pierce the diaphragm and cause an internal short circuit in the battery.
Therefore, methods to improve the low-temperature performance of LIBs are of great significance for promoting the use of electric vehicles in alpine regions.
This paper summarizes the methods to improve the low temperature performance of LiFePO4 batteries from the following four aspects:
1) Pulse current generates heat;
2) Use electrolyte additives to prepare high-quality SEI films;
3) Interface conductivity of surface coating modified LiFePO4 material;
4) Bulk conductivity of ion-doped modified LiFePO4 material.
Pulse current heat generation
During the charging process of LIBs rapidly heated by pulse current, the movement and polarization of ions in the electrolyte will promote the internal heat generation of LIBs. This heat generation mechanism can be effectively used to improve the performance of LIBs at low temperature. Pulse current refers to a current whose direction does not change and whose current intensity or voltage changes periodically with time. To rapidly and safely increase the battery temperature at low temperatures, how the pulsed current heats the LIBs is theoretically simulated, and the simulation results are verified by experimental tests on commercial LIBs. The difference in heat generation between continuous charging and pulsed charging is shown in Figure 1. As can be seen from Figure 1, the microsecond pulse time can promote more heat generation in the lithium battery.
In the above figure, Zhao et al. studied the excitation effect of pulse current on LiFePO4/MCNB batteries through the heat generated in pulse and continuous charging modes. Compared with the mode, the entire charging time is reduced by 36 min (23.4%), and the capacity is increased by 7.1% at the same discharge rate. Therefore, this charging mode is beneficial to fast charging of low-temperature LiFePO4 batteries.
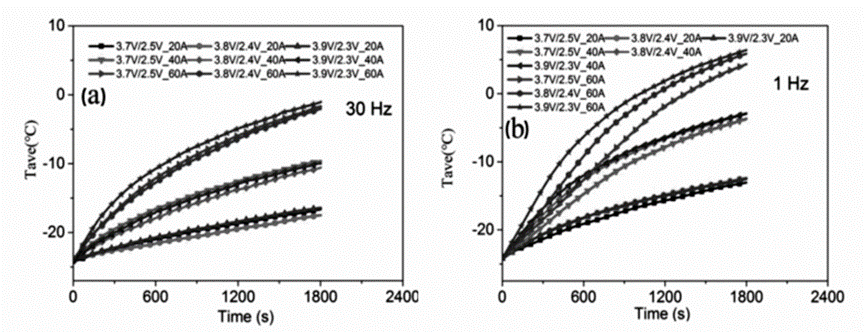
The influence of pulse current heating method on the low temperature battery life (health state) of LiFePO4 power lithium-ion battery was studied. They respectively studied the influence of pulse current frequency, current intensity and voltage range on battery temperature, as shown in the figure below. High current intensity, lower frequency and wider voltage range enhance the heat accumulation and temperature rise of LIBs. In addition, after 240 heating cycles (each cycle equal to 1800 s of pulsed heating at -20°C), they evaluated the health of LIBs after pulsed current heating by studying the cell capacity retention and electrochemical impedance, and analyzed by SEM and EDS The changes of the surface morphology of the negative electrode of the battery were studied, and the results showed that the pulse current heating does not increase the deposition of lithium ions on the surface of the negative electrode, so the pulse heating will not exacerbate the risk of capacity decay and lithium dendrite growth caused by lithium deposition.
Electrolyte modification of SEI membranes to reduce charge transfer resistance at the electrolyte-electrode interface
The low-temperature performance of lithium-ion batteries is closely related to the ion mobility in the battery. The effect of carbonate-based electrolyte (1 mol/L LiPF6/EC+DMC+DEC+EMC, with a volume ratio of 11:3) on the low-temperature performance of LiFePO4 commercial lithium batteries was studied. When the operating temperature is lower than -20 °C, the electrochemical performance of the battery decreases significantly, and electrochemical impedance spectroscopy (EIS) tests show that the increase in charge transfer resistance and the decrease in lithium ion diffusion capacity are the main factors for the degradation of battery performance. Therefore, it is expected to improve the low-temperature performance of LiFePO4 batteries by changing the electrolyte to enhance the reactivity of the electrolyte-electrode interface.
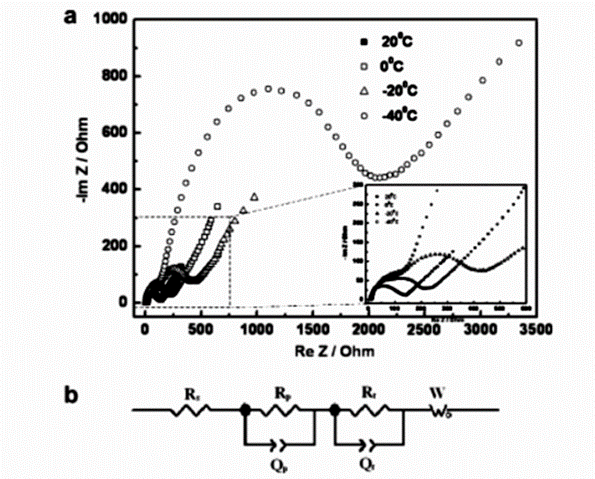
Above (a) EIS of LiFePO4 electrode at different temperatures; (b) Equivalent circuit model fitted by LiFePO4 EIS
In order to find an electrolyte system that can effectively improve the low-temperature electrochemical performance of LiFePO4 batteries, Zhang et al. tried adding LiBF4-LiBOB mixed salts to the electrolyte to improve the low-temperature cycling performance of LiFePO4 batteries. Notably, the optimized performance was achieved only when the molar fraction of LiBOB in the mixed salt was less than 10%. Zhou et al. dissolved LiPF4(C2O4)(LiFOP) into propylene carbonate (PC) as an electrolyte for LiFePO4/C batteries and compared it with the commonly used LiPF6-EC electrolyte system. It was found that the first cycle discharge capacity of LIBs decreased significantly when the battery was cycled at low temperature; meanwhile, the EIS data indicated that the LiFOP/PC electrolyte improved the low-temperature cycling performance of LIBs by reducing the internal impedance of LIBs.
Li et al. studied the electrochemical performance of two lithium difluoro(oxalate)borate (LiODFB) electrolyte systems: LiODFB-DMS and LiODFB-SL/DMS, and compared the electrochemical performance with the commonly used LiPF6-EC/DMC electrolyte, and found that LiODFB-SL/DMS and LiODFB-SL/DES electrolytes can improve the cycling stability and rate capability of LiFePO4 batteries at low temperature. EIS study found that LiODFB electrolyte is conducive to the formation of SEI film with lower interfacial impedance, which promotes the diffusion of ions and the movement of charges, thereby improving the low-temperature cycling performance of LiFePO4 batteries. Therefore, a suitable electrolyte composition is beneficial to reduce the charge transfer resistance and increase the diffusion rate of lithium ions at the electrode material interface, thereby effectively improving the low temperature performance of LIBs.
Electrolyte additives are also one of the effective ways to control the composition and structure of SEI films, thereby improving the performance of LIBs. Liao et al. studied the effect of FEC on the discharge capacity and rate performance of LiFePO4 batteries at low temperature. The study found that after adding 2% FEC to the electrolyte, LiFePO4 batteries showed higher discharge capacity and rate performance at low temperature. SEM and XPS showed the formation of SEI, and EIS results showed that the addition of FEC to the electrolyte can effectively reduce the impedance of LiFePO4 batteries at low temperature, so the improvement of battery performance is attributed to the increase of ionic conductivity of SEI film and the polarization of LiFePO4 electrode. reduce. Wu et al. used XPS to analyze the SEI film and further studied the related mechanism. They found that when FEC participated in the interface film formation, the decomposition of LiPF6 and carbonate solvent was weakened, and the content of LixPOyFz and carbonate substances produced by solvent decomposition decreased. Thereby, the SEI film with low resistance and dense structure is formed on the surface of LiFePO4. As shown in Fig. 4, after adding FEC, the CV curves of LiFePO4 show that the oxidation/reduction peaks are close together, indicating that the addition of FEC can reduce the polarization of the LiFePO4 electrode. Therefore, the modified SEI promotes the migration of lithium ions at the electrode/electrolyte interface, thereby enhancing the electrochemical performance of LiFePO4 electrodes.
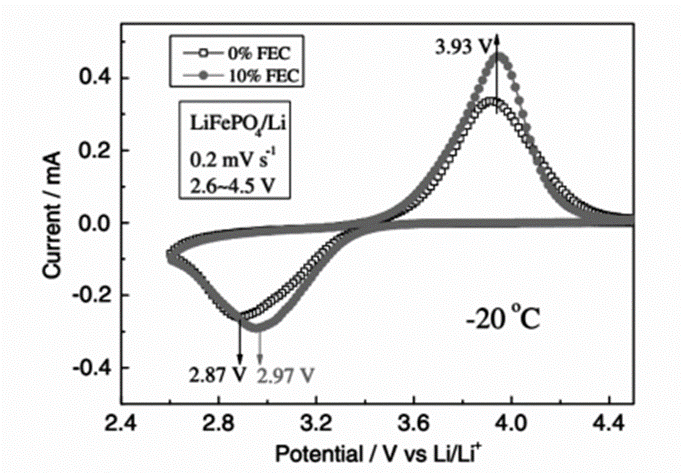
The above figure shows the cyclic voltammograms of LiFePO4 cells in electrolytes with volume fractions of 0% and 10% FEC at -20 °C
In addition, the study also found that the addition of butyl sultone (BS) to the electrolyte has a similar effect, that is, to form an SEI film with a thinner structure and lower impedance, and improve the migration rate of lithium ions when passing through the SEI film. Therefore, BS The addition of LiFePO4 significantly improves the capacity and rate performance of LiFePO4 batteries at low temperature.
Surface coating with conductive layer to reduce surface resistance of LiFePO4 material
One of the important reasons for the performance degradation of lithium batteries in low temperature environment is the increase of impedance at the electrode interface and the decrease of ion diffusion rate. LiFePO4 surface coating conductive layer can effectively reduce the contact resistance between electrode materials, thereby improving the diffusion rate of ions in and out of LiFePO4 at low temperature. As shown in Fig. 5, Wu et al. used two carbonaceous materials (amorphous carbon and carbon nanotubes) to coat LiFePO4 (LFP@C/CNT), and the modified LFP@C/CNT had excellent low-temperature performance. The capacity retention rate is about 71.4% when discharged at -25°C. EIS analysis found that this improvement in performance is mainly due to the reduced impedance of the LiFePO4 electrode material.
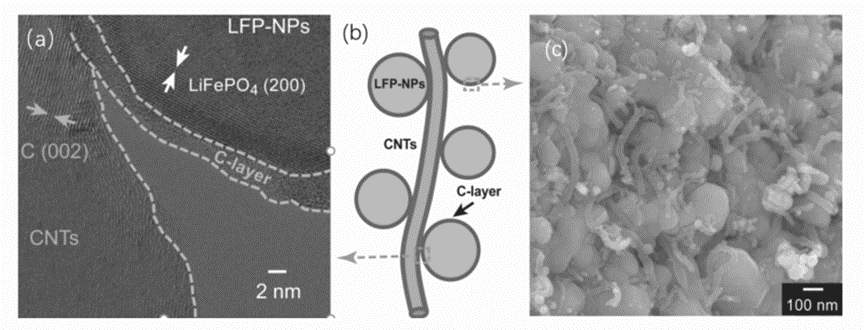
The HRTEM image (a), structural schematic diagram (b) and SEM image of the LFP@C/CNT nanocomposite above
Among many coating materials, metal or metal oxide nanoparticles have attracted the attention of many researchers due to their excellent electrical conductivity and simple preparation method. Yao et al. studied the effect of CeO2 coating on the performance of LiFePO4/C battery. In the experiment, CeO2 particles were uniformly distributed on the surface of LiFePO4. The kinetics are significantly improved, which is attributed to the improved contact between the electrode material and the current collector as well as the particles, as well as the increased charge transfer in the LiFePO4-electrolyte interface, which reduces the electrode polarization.
Similarly, taking advantage of the good electrical conductivity of V2O3, it was coated on the surface of LiFePO4, and the electrochemical properties of the coated samples were tested. The research on lithium ions shows that the V2O3 layer with good conductivity can significantly promote the lithium ion transport in the LiFePO4 electrode, thus the V2O3 modified LiFePO4/C battery exhibits excellent electrochemical performance in low temperature environment, as shown in the following figure .
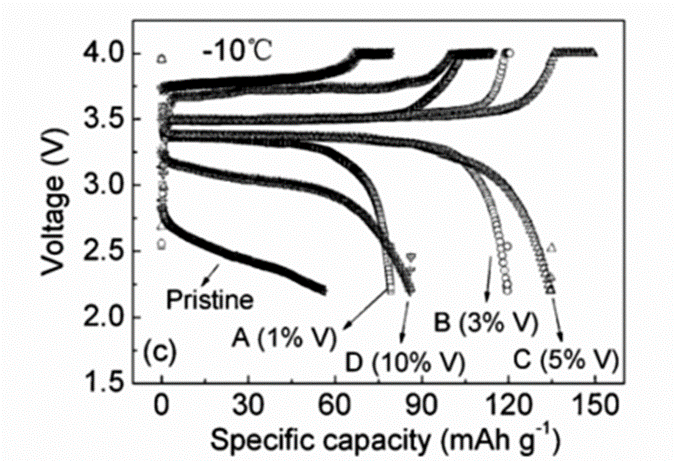
The above graph shows the cycling performance of LiFePO4 coated with different contents of V2O3 at low temperature
The surface of LiFePO4 material was coated with Sn nanoparticles by a simple electrodeposition (ED) process, and the effect of Sn coating on the electrochemical performance of LiFePO4/C cells was systematically investigated. SEM and EIS analysis showed that the Sn coating enhanced the contact between LiFePO4 particles, and the material had lower charge transfer resistance and higher lithium diffusion rate at low temperature.
Therefore, the Sn coating improves the specific capacity, cycling performance, and rate capability of LiFePO4/C cells at low temperature. In addition, Tang et al. used aluminum-doped zinc oxide (AZO) as a conductive material to coat the surface of LiFePO4 electrode material. The electrochemical test results show that the AZO coating can also greatly improve the rate capability and low temperature performance of LiFePO4, which is due to the conductive AZO coating increasing the electrical conductivity of the LiFePO4 material.
Bulk Doping Reduces Bulk Resistance of LiFePO4 Electrode Materials
Ion doping can form vacancies in the LiFePO4 olivine lattice structure, which promotes the diffusion rate of lithium ions in the material, thereby enhancing the electrochemical activity of LiFePO4 batteries. The lanthanum and magnesium doped Li0.99La0.01Fe0.9Mg0.1PO4/graphite aerogel composite electrode material was synthesized by solution impregnation process. The material exhibited excellent electrochemical performance at low temperature. The electrochemical impedance experiment results showed that, This superiority is mainly attributed to the enhanced electronic conductivity of the material by ion doping and graphite aerogel coating.
Conclusion and Outlook
This article briefly outlines 4 methods to improve the low temperature performance of lithium iron phosphate batteries:
- Pulse current generates heat;
- Electrolyte modified surface SEI film;
- Surface coating improves the surface conductivity of LiFePO4 material;
- Bulk ion doping improves the conductivity of LiFePO4 materials.
In the low temperature environment, the increase of interfacial resistance in LiFePO4 batteries and the growth of SEI film induced by lithium deposition are the main reasons for the deterioration of battery performance.
The pulsed current can accelerate the movement of the charges in the electrolyte to generate heat, which can rapidly heat up the LIBs. The use of low-impedance electrolyte systems or film-forming additives is conducive to the formation of dense and ultra-thin SEI films with high ionic conductivity, improving the reaction resistance of the LiFePO4 electrode-electrolyte interface, and reducing the negative effects of slow ion diffusion caused by low temperature.
There are two main ways to modify LiFePO4 materials: surface coating and ion doping.
The surface coating of LiFePO4 electrode material is conducive to improving the surface conductivity of the electrode material and reducing the contact resistance; while ion doping is beneficial to the formation of vacancies and valence changes in the lattice structure, broadening the ion diffusion channel, and promoting lithium ions and electrons in the material. migration rate.
Therefore, based on the above analysis, the key to improving the low-temperature performance of lithium iron phosphate batteries is to reduce the impedance inside the battery.
Keheng Self-Heating Battery
100AH 12V Low Temperature Heating Enable
Keheng New Energy’s Range Of Products
Escooter/ebike Battery
12V/24V LiFePO4 Battery
Portable power station
ESS energy storagy systems