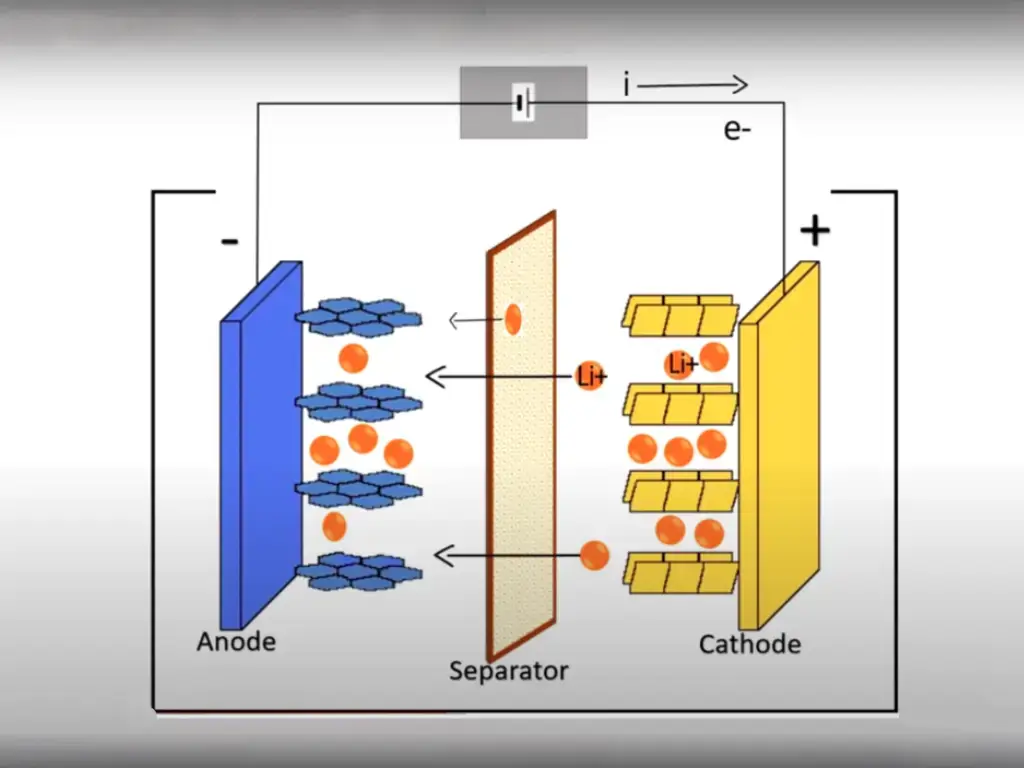
The characteristics of lithium-ion power batteries are significantly affected by ambient temperature, especially in low-temperature environments, where the available energy and power attenuate seriously, and long-term use in low-temperature environments will accelerate the aging of lithium-ion power batteries and shorten their service life.
With the rapid development of the new energy vehicle industry, some of its potential problems have begun to emerge.
For example, when an electric vehicle runs in a low temperature environment, the power failure of its main components such as its lithium-ion power battery and motor occurs.
It is understood that the driving range and charging and discharging performance of pure electric vehicles such as Tesla Models, Nissan Leaf, Chevrolet Volt, and BAIC New Energy EV series are severely challenged by low temperature environments.
During the promotion of electric vehicles, the cruising range, charging time and safety of use are mainly restricted by the characteristics of lithium-ion power batteries.
The characteristics of lithium-ion power batteries are significantly affected by ambient temperature, especially in low-temperature environments, where the available energy and power attenuate seriously, and long-term use in low-temperature environments will accelerate the aging of lithium-ion power batteries and shorten their service life.
The capacity and working voltage of the commonly used lithium-ion power battery for electric vehicles will be significantly reduced at -10°C, and the performance will be even worse at -20°C, which shows that its available discharge capacity drops sharply, and it can only maintain 30% of the specific capacity at room temperature. about.
It is also difficult to charge lithium-ion batteries in a low-temperature environment, and metal lithium is easily deposited on the surface of the negative electrode during charging. The growth of lithium dendrites will pierce the battery separator and cause an internal short circuit in the battery, which not only causes permanent damage to the battery, but also induces thermal runaway of the battery, which greatly reduces the safety of its use.
So, what factors restrict the low temperature performance of lithium ions?

Low temperature charging characteristics of lithium ion power batteries
If you want to understand the low temperature performance of lithium ion, you can analyze it by testing the low temperature characteristics of lithium ion power battery. To test the low-temperature characteristics of lithium-ion power batteries, lithium-ion power batteries of different specifications and materials can be used for testing, including low-temperature discharge, charging and AC impedance tests.
When the lithium-ion power battery starts charging, the battery terminal voltage rises instantly, and the lower the temperature is, the higher the starting voltage of the lithium-ion power battery charging will be. At low temperature, the terminal voltage rises faster, and will soon reach the cut-off voltage and enter the constant voltage charging stage.
As the temperature decreases, the constant current charging time of the lithium-ion power battery will be shortened, while the charging time in the constant voltage phase will be extended, and the total charging time will also become longer. Therefore, under the same charge, the charging time required for the lithium-ion power battery will be greatly increased.
In different temperature environments, the test results of the charging capacity of lithium-ion power batteries are divided into the charging capacity of the constant current stage and the charging capacity of the constant voltage stage. For the same battery with the same charging cut-off condition, as the temperature drops, the overall charge of the lithium-ion power battery shows a downward trend.
In the set charging mode, as the temperature decreases, the amount of electricity charged in the constant voltage stage of the lithium-ion power battery will increase. Therefore, the decrease in temperature leads to the reduction of the constant current charging capacity of lithium-ion power batteries, which mainly rely on constant voltage for charging. Long-term charging in the constant voltage stage will prolong the overall charging time of lithium-ion power batteries, reducing the efficiency of charging time, and long-term charging. The low temperature and constant voltage charging is also one of the reasons for the performance degradation of the side reaction of lithium-ion power batteries.
When lithium-ion power batteries are used at low temperatures, the energy and power characteristics are attenuated seriously. From a macro perspective, the low temperature performance of lithium-ion power batteries shows that with the decrease of temperature, the impedance of lithium-ion power batteries increases, the discharge voltage platform decreases, and the terminal voltage of the battery drops rapidly, resulting in a large amount of available capacity and power. attenuation.
Lithium-ion power batteries are not only difficult to achieve high current discharge at low temperatures, but also due to the increase in battery impedance, the charging voltage rises rapidly, shortening the time for the battery to reach the charging protection termination voltage, so there are disadvantages of difficult charging and low charging efficiency.
Microscopically, the low temperature characteristics of lithium-ion power batteries are mainly affected by the low ionic conductivity of the electrolyte inside the battery at low temperature, the reduction of the electrochemical reaction rate of the battery electrode at low temperature, the reduction of the conductivity of the SEI film on the surface of the graphite particles of the battery negative electrode at low temperature, and the low temperature. Under the constraints of factors such as the low solid-phase diffusion coefficient of lithium ions in the graphite material particles of the negative electrode of the battery.
Therefore, the performance of lithium-ion power batteries at low temperature is first related to the battery electrolyte. The electrolyte solvent of lithium-ion power battery not only directly affects the liquidus temperature range of the electrolyte, but also directly participates in the reaction of forming the SEI film.
The conductivity of the electrolyte decreases at low temperature, and the precipitated lithium metal easily reacts with the electrolyte due to low-temperature charging, resulting in further deterioration of the low-temperature performance of lithium-ion power batteries.
The increase in the resistance of the SEI film of the internal electrode of the battery at low temperature is another factor that deteriorates the low temperature performance of the lithium-ion power battery. At low temperature, the resistance of the SEI film of the internal electrode of the battery increases, and the available power of the lithium-ion power battery decreases.
During low-temperature charging, metallic lithium is precipitated on the surface of the negative electrode particles, and the reaction between the lithium metal and the electrolyte results in the thickening of the SEI film. On the one hand, the SEI film impedance of the battery is increased, and on the other hand, the reduction of available active lithium ions in the negative electrode can lead to irreversible capacity degradation of the lithium-ion power battery.
At low temperature, the electrochemical reaction rate of lithium-ion power battery decreases, and the internal resistance of charge transfer increases significantly. Compared with electrochemical ohmic internal resistance and SEI film impedance, the effect of temperature control on the electrochemical reaction process of the battery is more obvious, and the charge transfer internal resistance increases exponentially with the decrease of temperature. The main reason for the deterioration of the power performance of the ion power battery.
The reduction of the solid-phase diffusion coefficient of lithium ions in the anode graphite is also one of the main factors that lead to the deterioration of low-temperature performance of lithium-ion power batteries. The reduction of the solid-phase diffusion coefficient of lithium ions in the anode graphite at low temperatures is the main rate-controlling step that leads to the deterioration of the capacity characteristics of lithium-ion power batteries.
When the battery is charged at low temperature, the small diffusion coefficient will hinder the diffusion process of lithium ions in the negative electrode graphite, which will easily cause “lithium deposition” on the surface of the negative electrode particles, causing permanent damage to the battery.
Low temperature discharge characteristics of lithium ion power batteries
Take 18650 type nickel-cobalt-manganese system lithium-ion power battery, lithium iron phosphate system lithium-ion power battery, nickel-cobalt-manganese system lithium-ion power battery as an example, discharge test first. In the environment of 25 ℃, the three lithium-ion power batteries are charged with constant current and constant voltage to make the SOC (remaining power) reach 100%, and then stand for 4 hours at different temperatures, and wait for the battery temperature to reach the set temperature. Then, perform the corresponding test.
To study the discharge characteristics of the battery at low temperature, it is possible to use lithium-ion power batteries with different specifications under two different material systems to discharge voltages at different temperatures and different rates (1C, 2C), and use three lithium-ion power batteries with different characteristics. The rated capacity and current rate are used to uniformly analyze the characteristics of lithium-ion power batteries, as shown in Figure 3.
With the decrease of the ambient temperature, the discharge voltage of the battery shows a rapid downward trend, and the power characteristics of the lithium-ion power battery deteriorate. With the decrease of the temperature, the time for the lithium-ion power battery to reach the cut-off voltage is shortened, indicating that its available capacity is seriously attenuated. .
By comparison, it can be found that at the same temperature, the decay rate of the lithium iron phosphate system lithium-ion power battery is higher than that of the 18650 nickel-cobalt-manganese system lithium-ion power battery, which is determined by the material properties. The inherent low-temperature conductivity of lithium iron phosphate materials is poor, resulting in serious attenuation of low-temperature characteristics of lithium-ion power batteries.
Therefore, the lower the temperature, the greater the drop in the initial terminal voltage of the lithium-ion power battery. As the temperature decreases, the impedance of the lithium-ion power battery increases, resulting in an increase in the partial pressure of the internal resistance of the battery, so the terminal voltage of the battery decreases.
In the early stage of low-temperature discharge of the lithium-ion power battery, the terminal voltage has rebounded, which is mainly caused by the heat generation of the lithium-ion power battery during the discharge process.
To fully understand the influence of temperature and discharge rate on the power and capacity characteristics of lithium-ion power batteries, the available capacity ratio of two lithium-ion power batteries at different discharge rates and temperatures can be analyzed, as shown in Figure 6.
As the temperature decreases, the available capacity of the lithium-ion power battery will decay. Lithium-ion power battery capacity will drop significantly as the ambient temperature decreases. When the 18650 type nickel-cobalt-manganese system lithium-ion power battery has decayed to about 50% of the discharge capacity at 25 ℃ at 0.5C rate discharge and 1C rate discharge capacity at -30℃, the 2C constant current discharge capacity is 0.
According to the data comparison, at the same temperature, the decay rate of the lithium iron phosphate system lithium ion power battery is higher than that of the 18650 nickel cobalt manganese system lithium ion power battery, which is due to the poor low temperature conductivity of the lithium iron phosphate material.
The initial discharge terminal voltage of lithium-ion power batteries is not only affected by temperature, but also by the discharge rate. As the temperature drops, the initial discharge terminal voltage of the lithium-ion power battery continues to decrease, mainly because the temperature decreases, the internal resistance of the lithium-ion power battery increases, and the internal voltage of the lithium-ion power battery increases.
In addition, as the temperature decreases, the difference in the initial terminal voltage of the lithium-ion power battery at different rates is also more obvious. Temperature -30℃, the initial voltage of 0.5C rate discharge is only 6.8% lower than the initial voltage at 25℃, the initial voltage of 1C rate discharge is nearly 12.7% lower than the initial voltage of 25℃, 2C rate The initial discharge voltage dropped nearly 22.8% compared with the initial discharge voltage at 25 ℃.
At low temperature and high rate discharge, the output voltage of the lithium-ion power battery is also seriously attenuated, which affects the power output of the lithium-ion power battery. Under vehicle operating conditions, it mainly affects the acceleration and climbing characteristics of the vehicle.
Low temperature electrochemical impedance characteristics of lithium ion batteries
Electrochemical impedance spectroscopy (EIS), also known as AC impedance spectroscopy, is to measure the change with frequency by applying a small amplitude sinusoidal AC signal (voltage or current) to the electrochemical system in a certain frequency range. A method for the ratio of AC voltage to current signals.
This method can obtain more information on electrode interface structure and kinetics than other conventional electrochemical methods, so it is widely used to study the internal mechanism of Li-ion batteries.
High-frequency ohmic impedance increases with decreasing temperature; high-frequency and mid-frequency impedance gradually expands with decreasing temperature. Therefore, the film resistance of the solid-liquid interface and the internal resistance of charge transfer will increase.
At low temperature, for the 18650 type nickel-cobalt-manganese system lithium-ion power battery, the diffusion will disappear, and at -20 ℃, the impedance will increase to several times that at room temperature.
Therefore, the ultra-high frequency region (above 10 kHz) represents the transport of electrons through the particles of the active material and the transport of lithium ions in the electrolyte between the particles of the active material, which is represented as the intersection of the spectrum and the real axis on the EIS spectrum, Defined as the electrochemical ohmic internal resistance R0.
The diffusion and migration of lithium ions through the SEI film on the surface of the active material particles in the high-frequency region appears as a semi-circular arc on the impedance map. This process is equivalently replaced by the RSEI/CSEI parallel structure in the impedance model.
The impedance arc related to the electrochemical reaction in the intermediate frequency region includes two processes of charge migration and electric double layer charge and discharge. The charge transfer process occurs at the mutual interface of the solid-phase electrode and the electrolyte. This process follows Faraday’s law, so it is also called a Faraday process.
In the process of charge migration, the transfer speed of charge is reflected by the Faraday current. Generally, the charge migration process can be equivalent to a pure resistance, which is called charge migration internal resistance or Faraday resistance, and is represented by R ct.
The charge-discharge process of the electric double layer is also called the Faraday process. This process also occurs at the junction of the solid-phase electrode and the liquid-phase electrolyte interface, forming a physical structure similar to capacitance, thereby forming the interface electric double layer of the electrode. Capacitance Cdl represents.
The low frequency region is mainly due to the diffusion process of lithium ions in the active material particles. When the electrochemical reaction occurs, the Faradaic current flows through the interface between the solid-phase electrode and the electrolyte, resulting in the consumption of reactants and the accumulation of products on the interface, resulting in a concentration difference between the solid and liquid phases.
According to the porous electrode theory, the solid-phase electrode is assumed to be spherical particles with a certain porosity. As the reaction continues, the accumulation of substances inside the particles will increase, the concentration gradient of substances inside and outside the interface will decrease, and the diffusion rate of substances will decrease. slow.
When the substance on the electrode diffuses slowly into a steady state, stable concentration polarization occurs, that is, the polarization phenomenon caused by the difference in the lithium ion concentration distribution inside the battery.
Generally, the semi-infinite diffusion impedance Weber impedance ZW can be used to represent the diffusion process. Considering the geometrical factors of the electrode surface and the existence of adsorption, it is also represented by a constant phase element, which is represented by the symbol ZD.
Because the test range of EIS is 100 kHz – 0.01 Hz, no changes in the crystal structure of active material particles in the very low frequency region or the semicircle associated with the formation of new phases can be observed in the EIS spectrum. As shown in a in Figure 9, with the aid of the AC impedance spectrum analysis software ZView, the battery impedance parameters R0, RSEI and Rct are fitted and identified, and the three impedance values can be calculated and obtained according to the horizontal axis of the impedance spectrum.
The impedance will increase with the decrease of temperature, among which R0 and RSEI change relatively smoothly with the temperature, and the impedance value increases less with the decrease of temperature. But Rct will increase substantially with decreasing temperature. Because R0 and RSEI are mainly affected by the ionic conductivity in the electrolyte, the law of temperature change is similar to that of the electrolyte ionic conductivity with temperature.
In general, in order to solve the problems of new energy vehicles operating in low temperature environments, we must start with the performance of lithium-ion power batteries.
Aiming at the unfavorable factors of low temperature of lithium battery, Keheng lithium battery engineer team has developed the battery self-heating function in low temperature and extremely cold environment, which can effectively resolve this defect of lithium battery. Self-heating is an optional function of lithium iron phosphate deep cycle battery. All Keheng lithium iron phosphate batteries can be equipped with self-heating function, as well as Bluetooth function and BMS system mobile phone APP monitoring function.
Keheng self-heating battery
100AH 12V Low Temperature Heating Enable