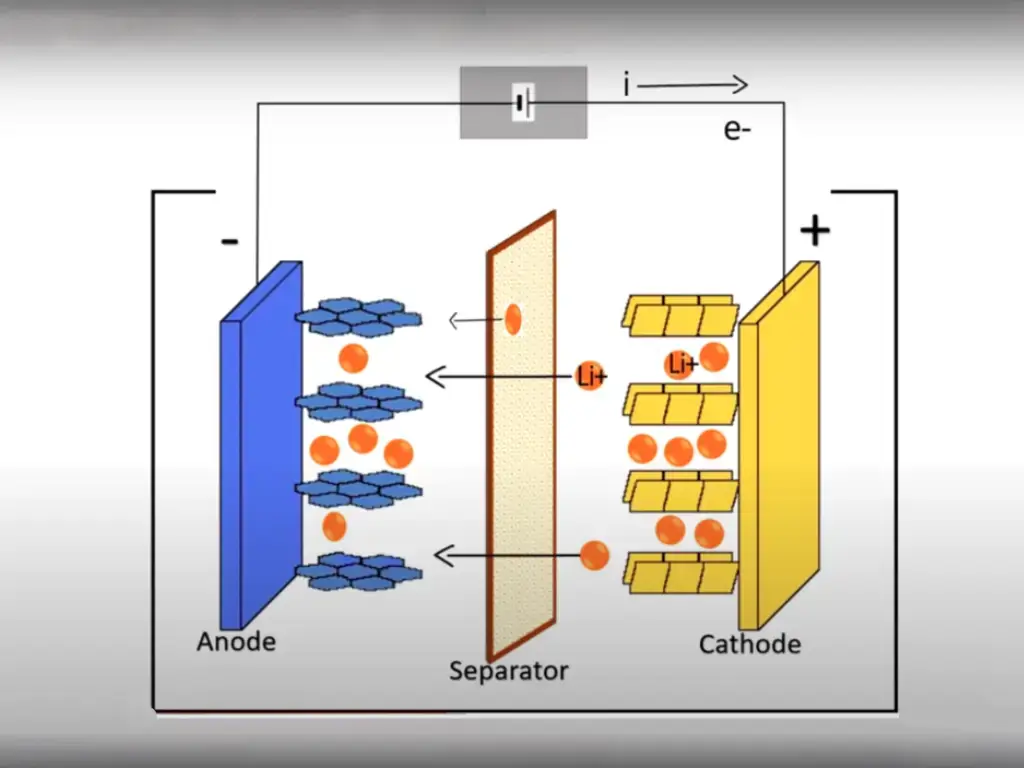
During the first charging process of the polymer lithium battery, the organic electrolyte will be reduced and decomposed on the surface of the negative electrode such as graphite to form a solid electrolyte phase interface (SEI) film, which permanently consumes a large amount of lithium from the positive electrode, resulting in the Coulombic efficiency (ICE) of the first cycle. ) is low, reducing the capacity and energy density of polymer lithium batteries.
Existing graphite materials have a first irreversible lithium loss of 5% to 10%, and for high-capacity anode materials, the first lithium loss is even higher (silicon has an irreversible capacity loss of 15% to 35%). To solve this problem, pre-lithiation technology has been studied. The electrode material is supplemented with lithium by pre-lithiation to offset the irreversible lithium loss caused by the formation of the SEI film, so as to improve the total capacity and energy density of the polymer lithium battery.
In this paper, the research progress of prelithiation technology in recent years is reviewed from the two directions of negative electrode lithium supplementation and positive electrode lithium supplementation.
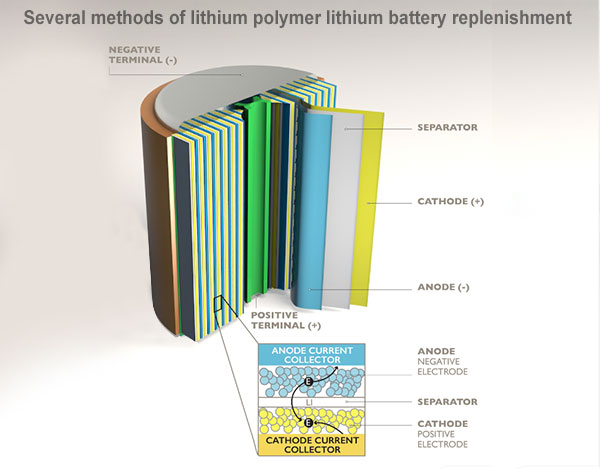
Negative Lithium Supplementation Technology
The common pre-lithiation method is to supplement the negative electrode with lithium, such as lithium foil supplemented with lithium, lithium powder supplemented with lithium, etc., which are all pre-lithiation processes that are currently being developed. In addition, there is a technique of pre-lithiation using lithium silicide powder and electrolytic lithium salt aqueous solution.
Lithium foil supplements lithium
Lithium foil replenishment is a technology that uses the self-discharge mechanism of polymer lithium batteries to replenish lithium. The potential of metallic lithium is -3.05V (vs. SHE, standard hydrogen electrode), the lowest among all electrode materials. Due to the existence of the potential difference, when the negative electrode material is in contact with the metallic lithium foil, the electrons move to the negative electrode spontaneously, accompanied by the intercalation of Li+ in the negative electrode.
Electrolyte was added dropwise to the negative electrode of silicon nanowires (SiNWs) grown on a stainless steel substrate, and then directly contacted with lithium metal foil for lithium supplementation.
The half-cell test of the negative electrode after lithium supplementation found that:
The open-circuit voltage (OCV) of the SiNWs without lithium supplementation is 1.55V, and the lithium intercalation specific capacity of the first 0.1 C discharge at 0.01-1.00 V is 3800 mAh/g;
The OCV of the SiNWs after lithium supplementation is 0.25 V, and the specific capacity of the first lithium intercalation is 1600 mAh/g. The changes in OCV and lithium intercalation specific capacity indicate that Si has partially reacted with Li after lithium supplementation.
The tin-carbon (Sn-C) negative electrode was directly contacted with the lithium foil infiltrated by the electrolyte for 180 min to supplement lithium. The irreversible specific capacity of Sn-C was reduced from 680mAh/g( 63% ) to 65 mAh /g( 14% ) after lithium supplementation when tested with half-cell at 80 mA/g at 0.01-2.00V.
The negative electrode and LiNi0. 45Co0. 1Mn1. 45O4 constitute a full battery, the ICE tested at 1. 0 C rate at 3. 1 ~ 4. 8 V is close to 100%, and the cycle is stable and the rate performance is good; 5. 0 C The discharge specific capacity reaches 110 mAh/g, which is only 14% lower than the discharge capacity at 0.2 C.
Although the pre-lithiation of the negative electrode can be achieved by direct contact with the lithium foil, the degree of pre-lithiation is not easy to precisely control. Insufficient lithiation can not fully improve ICE; and excessive lithium supplementation may form a metallic lithium coating on the surface of the negative electrode.
Using an external short circuit, the silicon oxide negative electrode (c-SiOx) is supplemented with lithium through lithium foil. The comparison experiment shows that the ICE can be maximized when the resistance in the external short circuit is 100 Ω and the short circuit time is 30min. Half-cell tests were performed on c-SiOx before and after lithium supplementation. The first 5 cycles of 0.07 C at 0.01 to 1.50 V showed that the Coulombic efficiencies of the electrodes before lithium supplementation were 73.6%, 94.7%, 96.6%, 97.5% and 98.5%. 0%;
The Coulombic efficiencies of the electrodes after lithium supplementation were 94.9%, 95.7%, 97.2%, 97.9% and 98.3%. The full battery was composed of c-SiOx and LiNi0. 8 Co0. 15 Al0. 05 O2, and the current of 10 mA/g was used to test at 2. 5 ~ 4. 2 V. 106.33 mAh /g increased to 165.09mAh /g, ICE increased from 58.85% to 85.34%.
The safety of lithium foil supplementation has been improved. The designed active material/polymer/lithium metal three-layer structure negative electrode can be stable in ambient air (relative humidity of 10% to 30%) for 30 to 60 minutes, which is enough for the negative electrode to carry out processing.
The three-layer structure is: a metal lithium layer by electrochemical deposition on the copper foil, the lithium layer is coated with a polymethyl methacrylate (PMMA) protective layer and an active material layer. By changing the thickness of the lithium layer, the degree of lithium supplementation can be controlled. After the electrolyte is injected into the battery to dissolve the PMMA, the lithium layer and the active material are in direct contact to complete the pre-lithiation. Tested at 0.1C at 0.01-1.00 V, the ICE increased from 92.0% to 99.7% using the graphite after lithium supplementation with the three-layer structure; after the pure silicon negative electrode was supplemented with lithium, there was almost no capacity loss during the first charge and discharge. Although the use of lithium foil for lithium supplementation has a good effect, the lithium supplementation process needs to be completed in a temporary battery or electrochemical device, which is difficult to scale up.
Stabilized Lithium Metal Powder (SLMP)
Lithium powder supplementation was proposed by FMC. The developed SLMP has a specific capacity of up to 3600 mAh/g, and the surface is coated with a 2% to 5% lithium carbonate (Li2CO3) thin layer, which can be used in a dry environment. There are two main ways to apply SLMP to negative electrode pre-lithiation: adding it during the slurry mixing process, or adding it directly to the surface of the negative electrode sheet.
Conventional polymer lithium battery anode slurry, using polyvinylidene fluoride (PVDF) / methylpyrrolidone (NMP) or styrene butadiene rubber (SBR) + carboxymethyl cellulose (CMC) / deionized water system, but SLMP and Polar solvents are incompatible and can only be dispersed in non-polar solvents such as hexane and toluene, so they cannot be directly added in the conventional slurry mixing process. L. Wang et al. used the SBR-PVDF/toluene system to directly mix SLMP in the graphite electrode slurry. First, graphite and PVDF were mixed in NMP solvent, and after drying, PVDF-coated graphite was formed; then SLMP, PVDF-coated graphite, and conductive carbon black were mixed in toluene; finally, SBR was added. After pre-lithiation of the negative electrode by SLMP, the ICE of the battery increased from 90.6% to 96.2% under the conditions of 0.01-1.00 V and 0.05 C.
Compared with adding it during the mixing process, it is simpler and easier to directly load SLMP onto the dry anode surface. The silicon (Si)-carbon nanotube (CNT) negative electrode was pre-lithiated using SLMP, and the SLMP/toluene solution with a mass fraction of 3% was dropped on the surface of the Si-CNT negative electrode. After the toluene solvent was volatilized, tableting and activation were performed. . After pre-lithiation, the first irreversible capacity of the negative electrode is reduced by 20% to 40%.
The SLMP was dispersed in a xylene solution containing 1% SBR/polystyrene to form a stable SLMP slurry. The SLMP slurry was coated on the surface of the dried negative electrode to realize the pre-lithiation of the negative electrode such as graphite and SiO. After pre-lithiation, the graphite|nickel-cobalt-manganese ternary material (NCM) full cell was tested at 0.1 C at 3.0~4.2 V, and the ICE increased from 82.35% to 87.80%; % increased to 88.12% after prelithiation.
Lithium silicide powder
Compared with the micron-sized SLMP, the size (100-200 nm) of nano-lithium silicide (LixSi) is smaller, which is more favorable for the dispersion in the negative electrode. In addition, LixSi is already in an expanded state, and the volume change during cycling will not affect the structure of the entire electrode.
At present, there are few studies on LixSi supplementary additives, and researches have been carried out on the improvement of LixSi’s lithium supplementation performance and stability. In an argon atmosphere, the Li2O-coated LixSi material was synthesized by the alloying reaction of silicon and metallic lithium at 200 ℃. The half-cell system was charged and discharged at 0.05 C at 0.01 to 1.00 V. After adding 15% LixSi, the ICE of silicon anode increased from 76% to 94%; the ICE of mesocarbon microspheres (MCMB) added 9% LixSi increased from 75% increased to 99%; the ICE of the graphite anode with 7% LixSi was increased from 87% to 99%.
Under the full-cell system, the ICE of graphite|LiFePO4 cells with 7% LixSi addition increased from 77.6% to 90.8%, and had higher capacity in subsequent cycling tests.
The synthesized LixSi has good performance of lithium supplementation, but it can only maintain relative stability in dry air. After being exposed to dry air with a dew point of -50 °C for 5 d, the capacity decays by 30%, and it is completely deactivated in the air environment. In order to improve the stability of LixSi, 1-fluorodecane can be used to reduce the particle surface to form a dense coating. After the coated LixSi was placed in dry air for 5 days, there was almost no attenuation. After being placed in air with a relative humidity of 10% for 6 h, the specific capacity was still as high as 1604 mAh / 0.01-1.00 V and 0.02 C. g, the capacity retention rate reaches 77%.
Adding 5% to the graphite anode for lithium supplementation, under the conditions of 0.005-1.500 V and 0.05 C, the ICE increased from 87.0% to 96.7%. In order to further improve the stability of LixSi, SiO and SiO2 can be used to replace Si as raw materials to synthesize LixSi-Li2O composite materials. After the composite was placed in air with a relative humidity of 40% for 6 h, the specific capacity was as high as 1240 mAh/g under the conditions of 0.01–1.00 V and 0.02 C. The LixSi-Li2O composites synthesized from the two raw materials showed excellent performance of lithium supplementation.
Electrolyzing lithium salt aqueous solution for lithium supplementation
Whether it is using lithium foil, SLMP or lithium silicide powder to supplement lithium, the use of metal lithium is involved. Lithium metal has high price, high activity, and is difficult to operate. Storage and transportation require high costs for protection. If the lithium replenishment process does not involve metallic lithium, the cost can be saved and the safety performance can be improved. Silicon is supplemented with lithium by electrolyzing Li2SO4 aqueous solution in an electrolytic cell. The sacrificial electrode is a copper wire immersed in Li2SO4. The lithium supplementation reaction is shown in formula (1):
Electrolyzed at a current of 1 A/g for 4.2 h, the MnOx|Si full cell after lithium supplementation was tested at 0.5 to 3.8 V, and the specific capacities of 0.5 C, 1.0 C, 2.0 C, 4.0 C and 8.0 C were 160 mAh/g, respectively , 136 mAh/g, 122 mAh/g, 108 mAh/g and 92 mAh/g.
Cathode Lithium Supplementation Technology
Compared with the difficult and high-input negative electrode lithium supplement, the positive electrode lithium supplement is much simpler. A typical cathode lithium supplement is to add a small amount of high-capacity material during the cathode slurry mixing process. During the charging process, Li+ is extracted from the high-capacity material to supplement the irreversible capacity loss of the first charge and discharge. At present, the materials used as cathode lithium supplementary additives mainly include: lithium-rich compounds, nanocomposites based on conversion reactions, and binary lithium compounds.
Lithium-rich compounds
The irreversible capacity loss of the Si-C| LiNi0. 5Mn1. 5O4 full cell is compensated by using the lithium-rich material Li1 + x Ni0. 5 Mn1. 5O4. The capacity retention rate of the battery using the hybrid cathode for 100 cycles at 3.00-4.78 V at 0.33 C was 75%, while that of the battery using the pure LiNi0.5 Mn1. 5 O4 cathode was only 51%. Furthermore, the energy density of the Si-C|LiNi0.5Mn1.5O4 battery using the hybrid cathode was increased by 25% compared to the graphite|LiNi0.5Mn1.5O4 battery.
Li2NiO2 can also be used as a positive lithium supplementary additive, but its stability in air is poor. Li2NiO2 was modified with aluminum isopropoxide, and an alumina-coated Li2NiO2 material that was stable in air was synthesized, and the lithium supplementation effect was excellent. The unadded LiCoO2|graphite full battery has an ICE of 92% at 2.75-4.20V and 0.2 C, and the battery with 4% Li2NiO2 added has almost no capacity loss, and the rate performance is not affected by additives.
Li5FeO4 (LFO) was added to the LiCoO2 cathode to compensate for the capacity loss of the hard carbon anode during the first charge. Half-cell tests show that the first charge-discharge (2.75-4.30V) specific capacity of LiCoO2 positive electrode with 7% LFO added at 0.1 C is 233 mAh /g and 160 mAh /g, respectively, and the irreversible capacity accounts for 31%, which is enough to compensate the 22% of hard carbon. First irreversible capacity loss. The results of the full-cell test (2.75-4.30 V, 0.05 C) showed that the LiCoO2|hard carbon full-cell with 7% LFO added had a 14% increase in reversible capacity, a 10% increase in energy density, and an improved cycle performance. The specific capacity retention after 50 cycles improved from less than 90% to more than 95%. For the LiCoO2 cathode with LFO added, the processes such as slurry mixing and coating need to be carried out in an inert atmosphere, and the stability of LFO in the air environment needs to be improved.
Nanocomposites based on transformation reactions
Although lithium-rich compounds have achieved certain effects as lithium-supplementing additives, the first lithium-replenishing effect is still limited by a low specific capacity. Due to the large charge/discharge voltage hysteresis, the nanocomposites based on the conversion reaction can contribute a large amount of lithium during the first charging process of the battery, while the lithium intercalation reaction cannot occur during the discharge process.
The properties of M/lithium oxide (Li2O), M/lithium fluoride (LiF), and M/lithium sulfide (Li2S) (M = Co, Ni and Fe) as cathode lithium supplementary additives were investigated. M/Li2O was synthesized by mixing MxOy and molten lithium under argon atmosphere. The synthesized nano-Co/nano-Li2O (N-Co/N-Li2O) composite was cycled at 50 mA/g at 4.1 to 2.5 V, with a specific capacity of 619 mAh/g for the first charge and only 10 mAh/g for discharge. ; After N-Co/NLi2O was exposed to ambient air for 8 h, the delithiation specific capacity was only 51 mAh/g smaller than the initial value, and after 2 d, the delithiation specific capacity was still 418 mAh/g, indicating that NCo/N -Li2O has good environmental stability and is compatible with the production process of commercial batteries. Similar to N-Co/N-Li2O, N-Ni/N-Li2O, N-Fe/N-Li2O also have high specific charge capacities (506 mAh/g and 631 mAh/g, respectively) and very low discharges The specific capacity (respectively 11 mAh/g and 19 mAh/g), the performance of lithium supplement is excellent.
LiF has high lithium content and good stability, and is a potential cathode lithium supplement material. The M/LiF nanomaterials constructed by the conversion reaction can overcome the problems of low electrical and ionic conductivity, high electrochemical decomposition potential, and harmful decomposition products of LiF, making LiF an excellent cathode lithium supplementary additive. The delithiation specific capacity of LiF/Co at 4.2-2.5 V is as high as 520 mAh/g, and the lithium intercalation specific capacity is only 4 mAh/g, indicating that the lithium replenishment capacity of LiF/Co reaches 516 mAh/g. The delithiation specific capacity of LiF/Fe at 4.3-2.5 V is 532 mAh/g, and the lithium intercalation specific capacity is 26 mAh/g, indicating that the lithium replenishment capacity of LiF/Fe reaches 506 mAh/g. The LiFePO4|Li half-cell with 4.8% LiF/Co added has a specific capacity of 197 mAh/g for the first charge at 2.5-4.2 V at 0.1 C, which is 20.1% higher than that of the 164 mAh/g without LiF/Co. The specific capacities are similar, and the cycle stability is not affected by additives.
The theoretical capacity of Li2S reaches 1166 mAh/g, but as a lithium supplementary additive, there are still many problems to be solved, such as compatibility with electrolyte, poor insulation, and poor environmental stability. It has been found that these problems can be solved by introducing metals into Li2S to form L2S/M composites. The Li2S/Co synthesized from CoS2 and Li metal has a lithium-replenishing capacity of 670 mAh/g. The LFP electrode with added 4.8% Li2S/Co has a specific capacity of 204 mAh/g for the first charge at 2.5-4.2 V with 0.1 C, which is 42 mAh/g higher than that of the electrode without addition. The Li2S/Fe synthesized from FeS2 and metal Li has a lithium replenishment capacity of 480 mAh/g. Although the lithium replenishment capacity is lower than that of Li2S/Co, the raw material FeS2 is abundant and cheap, so the commercial application prospect of Li2S/Fe is better. Although the lithium-rich compound has a higher lithium replenishment capacity, the nanocomposite based on the conversion reaction will remain inactive metal oxides, fluorides and sulfides after the first lithium replenishment, which reduces the energy density of the battery.
Binary Lithium Compounds
Compared with lithium-rich oxides (about 300 mAh/g) and conversion reaction composites (500-700 mAh/g), the theoretical specific capacity of binary lithium compounds is much higher. The theoretical specific capacities of Li2O2, Li2O, and Li3N reach 1168 mAh/g, 1797 mAh/g, and 2309 mAh/g, respectively. Only a small amount of addition is needed to achieve a similar lithium supplementation effect. Theoretically, the residues of these materials after lithium supplementation are O2, N2, etc., which can be exhausted during the formation of the SEI film in the battery.
The commercial Li3N was ground into powder with a particle size of 1 to 5 μm, which was used as a lithium supplementary additive. In the half-cell system, LiCoO2 electrodes with 1% and 2% Li3N added, the specific capacities of the first charge at 0.1 C at 3.0-4.2 V were 167.6 mAh/g and 178.4 mAh/g, respectively, which was 18.0 mAh/g higher than that of pure LiCoO2. g, 28.7 mAh/g. The discharge capacity of the LiCoO2 | SiOx /C@Si full cell with the addition of 2% Li3N at 0.5 C at 1.75 to 4.15 V increased by 11% compared with the full cell without the addition of the additive. In order to solve the problem of the conductivity of the mixed electrode, Li3N is deposited on the surface of the LiCoO2 electrode, which can reduce the influence on the conductivity of the electrode. The full battery with 5% additive deposited on the surface of the positive electrode has a discharge specific capacity of 126.3 mAh /g, which is 14.6 mAh /g higher than that of the battery without additives, and the rate performance is similar to the cycle performance.
In addition, loading Li3N on the dry electrode surface can avoid the incompatibility of Li3N with slurry solvents such as methylpyrrolidone.
The commercial Li2O2 was mixed with LiNi0. 33 Co0. 33 Mn0. 33 O2 (NCM) to compensate for the lithium loss during the first charge of the graphite anode. The NCM in the hybrid electrode plays the dual role of active material and catalyst. For efficient catalytic decomposition of Li2O2, 1% NCM-6 h (NCM obtained by ball milling for 6 h) was added to the cathode. The graphite|NCM/NCM-6 h/2% Li2O2 full cell was charged and discharged at 2.75 to 4.60 V, and the first reversible specific capacity at 0.1 C was 207.1mAh/g, which was 7.8% higher than that of the graphite|NCM full cell; 0.3 C reversible specific capacity It is 165.4 mAh/g, which is 20.5% higher than the graphite|NCM full battery. The test shows that the oxygen released by the decomposition of Li2O2 will consume the limited Li+ in the full cell, resulting in obvious capacity decay in the full cell with Li2O2 addition, but the capacity can be recovered after the gas is exhausted. The first charging of the battery in the actual production process is carried out in an open system, and the gas generated by the formation of the SEI film and some side reactions will be discharged before sealing, so the impact caused by the release of O2 can be reduced.
The effect of micron-sized Li2O as a positive lithium supplementary additive was studied, and the SiO-SnCoC|Li1.2Ni0.15Mn0.55Co0.1O2 full cell with 20% Li2O added, the first discharge specific capacity at 10 mA/g at 2.0 ~ 4.5 V cycle From 176 mAh/g to 254 mAh/g. The experimental results show that the lithium-rich material Li1.2Ni0.15Mn0.55Co0.1O2 plays the dual roles of active material and catalyst.
Conclusion
Comparing the two lithium-replenishing methods for polymer lithium batteries, the negative-electrode lithium-replenishing route has high capacity for lithium-replenishing reagents (lithium foil, lithium powder, and lithium silicide powder), but the operation is complicated and requires high environmental requirements; by adding lithium-replenishing additives to the positive electrode The positive electrode lithium supplementation route is superior in high safety and stability, and good compatibility with the existing battery production process.
Keheng Self-Heating Battery
100AH 12V Low Temperature Heating Enable



1 thought on “Several methods of polymer lithium battery replenishment”
Hello, Thank you for sharing this informative post! I really enjoyed reading about your perspective on this topic and appreciated the insight you provided. I found the information you presented very helpful and beneficial to me. I look forward to reading more posts from you in the future! I hope you can also read my writing related to health, and I hope my writing can also add insight to all of us.