Among the deep cycle batteries, rechargeable lead-acid batteries come in many types and are often used to start the vehicle and operate as an engine, as well as for emergency lighting. AGM batteries have been around since the 1980s, and perhaps most people don’t know what AGM stands for. In this issue, we will discuss what is special about AGM batteries and how to use our AGM batteries reasonably!

What is an AGM battery?
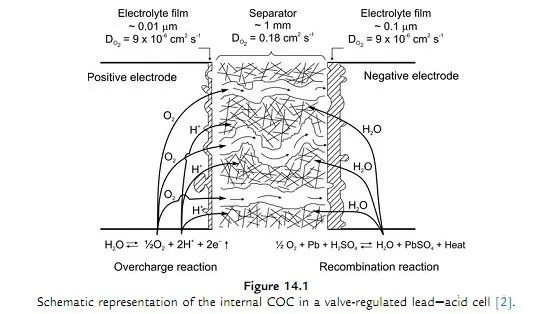
The full name of AGM battery is absorption glass mat battery, which belongs to VRLA battery (valve regulated lead-acid battery). The biggest feature of AGM batteries is the glass fiber mesh on the battery plates, which is used to hold the electrolyte and separate the plates. Secondly, there are safety valves and other components.
The Birth of AGM Batteries
AGM batteries originated in 1972, the first AGM battery produced by the patented Gates Rubber Company called Cyclon, now produced by EnerSys. AGM batteries became popular in 1980 for powering motorcycles, military, aircraft and submarines, and as a maintenance-free replacement for traditional flooded lead-acid batteries. In the mid-1980s, two British companies, Chloride and Tungstone, simultaneously introduced ten-year-life AGM batteries with capacities up to 400 Ah, driven by British Telecom’s battery specification to support new digital switching.
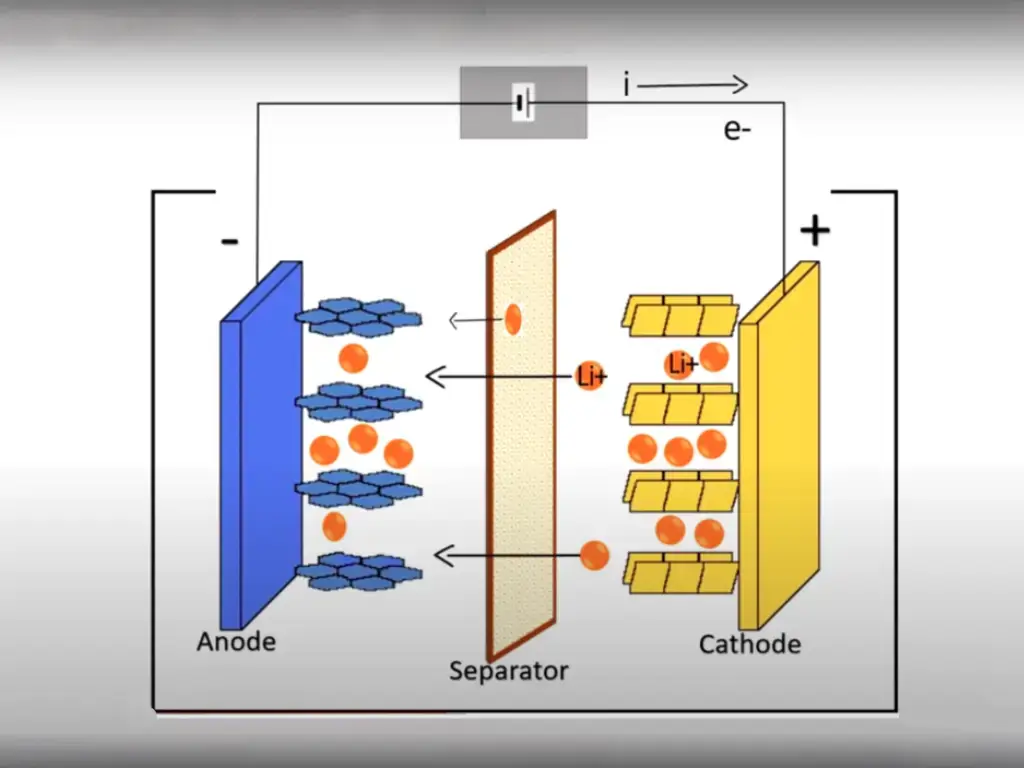
How AGM Batteries Work?
Lead acid battery electrode
The charging reaction :(+) PbO2 + 3H+ + HSO4- + 2e ≒ PbSO4 + 2H2O
When the battery is charged, the positive electrode is converted from lead sulfate (PbSO4) to brown lead dioxide (PbO2), and the negative electrode is converted from PbSO4 to gray lead Pb. As the charging process progresses, the positive electrode potential increases gradually and the negative electrode potential decreases.
At the end of charging, the electrolysis reaction of water occurs, the positive electrode begins to generate oxygen, and the negative electrode does not generate hydrogen due to excess active material and the addition of metals with high hydrogen evolution potential (such as calcium, cadmium).
The oxygen generated by the positive electrode is transmitted to the negative electrode through the AGM diaphragm, and is combined with the negative electrode to synthesize lead oxide. The lead oxide is combined with sulfuric acid to generate water to realize the internal water circulation. Thus, the entire lead-acid battery is sealed.
Be careful:Sealed lead-acid batteries must require constant voltage charging, because in the charging process, in order to ensure that only a small amount of oxygen is generated to ensure that it can be transferred to the negative electrode in time for recombination to avoid water loss. If the voltage is too high at this time, a large amount of oxygen will be produced, which will cause the oxygen to be too late to combine and the internal pressure will increase sharply. Finally, the safety valve will be opened to cause water loss, which will affect the battery life.
Discharge(-)Pb + HSO4 – ≒ PbSO4 + H+ +2e
The positive electrode of the battery discharge is changed from lead dioxide to lead sulfate, and the negative electrode is changed from spongy lead to lead sulfate.
During this process, the battery voltage gradually decreased, and the sulfuric acid concentration continued to decrease. At the end of discharge, the ohmic resistance of the electrode increases rapidly due to the gradual accumulation of lead sulfate, a bad conductor generated by the positive and negative electrodes. At the same time, the hydrogen ions diffuse slowly after the sulfuric acid concentration decreases, resulting in a rapid drop in the battery voltage. At this time, the discharge should be terminated, otherwise excessive discharge.
Be careful:The harm of battery over-discharge is that part of lead sulfate cannot be converted and recovered normally when recharged, and the battery capacity will decrease during the next discharge. Repeated over-discharge will cause the battery capacity to decay rapidly and the service life to be shortened significantly.
The difference between AGM battery and submerged lead-acid battery
AGM battery electrolytes are held in glass mats rather than freely immersed in the plates. Very thin fiberglass is woven into the mat to increase the surface area, allowing itself to have enough electrolyte to last its life. The fibers that make up the fine glass mat do not absorb and are not affected by acidic electrolytes. These pads are wrung out 2-5% after soaking in acid before finishing manufacturing.
The plates in AGM batteries can be of any shape. Including flat, curved, curled various shapes. According to the Battery Council International (BCI) battery code specification, both deep cycle and start-type AGM batteries are built into rectangular housings.
Advantages and disadvantages of AGM batteries
AGM battery advantages
1. Has 2-3 times the service life of submerged lead-acid batteries (500-1300, depending on the depth of discharge DOD).
2. AGM has more power than flooded battery and is easier to start the car engine.
3. The charging speed is faster, 5 times the charging speed of the flooded battery.
4. Higher capacitance stability over the entire service life.
5. Good resistance to both low temperature and high temperature.
6. Sealed design, no need to worry about the orientation of the installation, reducing the risk of environmental pollution
7. Simple maintenance, in a wide range, with lower self-discharge of traditional batteries.
AGM battery disadvantages
1.Due to the sealed design, the amount of electrolysis is small, and the thick plate design makes the utilization rate of active materials lower than that of open-type batteries, and the discharge capacity is about 10% lower than that of open-type batteries, which is lower than that of gel-sealed lead-acid batteries.
2.Despite its maintenance-free reputation, there is still a risk of air leaks, liquid leaks, swelling and even explosions (thermal runaway) if not properly maintained.
3.It has to be admitted that the price of AGM car batteries is twice that of flooded batteries.
4.After the initial high-capacity charge, they take longer to absorb the charge phase in the middle when the current is gradually reduced, at which time the charge acceptance rate of the lead-acid battery is gradually reduced, and the battery will not be able to accept the higher C-rate.
The difference between AGM battery and VRLA battery
AGM battery is a member of VRLA valve-regulated lead-acid battery, followed by gel battery. This type of battery has a characteristic, they are all by absorbing a limited amount of electrolyte plate separator or forming a gel; through the ratio of negative plate and positive plate to promote oxygen recombination in the cell; each VRLA battery Every battery has a pressure relief valve, which is triggered when the battery starts to charge and the oxygen pressure starts to be generated inside the battery.
VRLA batteries typically have a built-in gas diffuser in the battery cover to safely disperse excess hydrogen gas from overcharging, while allowing for orientation in any way, not actually being permanently sealed.
In contrast, gel batteries form a thick, putty-like gel by adding silica dust to the electrolyte. AGM cells have a fiberglass mesh between the panels to hold the electrolyte and separate the panels. Their biggest feature is that they are “maintenance-free” and can be installed in any direction.
What do I need to pay attention to when using the charger for AGM batteries?
Any standard regular battery charger will not charge the AGM. Unlike standard flooded charging requirements, AGM batteries require a specific charger because it requires a more stable charge and cannot sustain the high charging speeds used by standard chargers.
If you charge with the wrong type of charger, the battery may be undercharged or overcharged. Undercharging can lead to sulfation of the battery plates, reducing their ability to accept a full charge. Overcharging can burn the components inside the battery, shortening its life or draining it completely.

Application of AGM battery
AGM is generally used to start or stop a car’s engine. Since the car is equipped with more electronic equipment, the AGM battery reduces the weight of the car and provides electrical needs. Many motorcycles and all-terrain vehicles on the market today use AGM batteries to reduce the possibility of acid spills after cornering, vibration, or an accident.
Since AGM batteries lack free electrolytes, they are ideal for powering remote sensors such as ice monitoring stations. In some recreational marine applications, AGM can still be used as a deep cycle battery, which can be a good replacement for traditional flooded batteries, a good replacement for RV travel, marine marine batteries, and is also commonly used in some off-grid solar and wind power generation and storage.